| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
HIV-1;
HSV-1;
BoHV-1;
SARS-CoV-2;
|
|---|---|
| 体外研究 (In Vitro) |
伊维菌素 (MK-933) 在亚微摩尔范围内 (EC50=250 nM) 快速、可逆地发挥作用,增加振幅并延迟 ATP 诱发的 P2X4 通道电流的失活。在不改变 P2X4 通道离子选择性的情况下,伊维菌素 (MK-933) ) 以与使用和电压无关的方式显着增强 ATP 和典型低效激动剂 a,b-亚甲基-ATP 的效力[1]。
伊维菌素 (MK-933) 会导致膜超极化和肌肉紧张通过激活寄生虫神经和肌肉中的谷氨酸门控氯通道来麻痹寄生虫[2]。 Impα/β1 与 NS5 的结合被伊维菌素 (MK-933) 强烈抑制 (IC50=17 μM),但不被伊维菌素 (MK-933) 强烈抑制。 Impβ1 本身的结合。伊维菌素 (MK-933) 对登革热病毒和 HIV-1 表现出强大的抗病毒活性,这两种病毒都严重依赖于 NS5(非结构蛋白 5)的导入 α/β 核导入分别是聚合酶和 HIV-1 整合酶蛋白[3]。 |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Moderately well absorbed. Improved absorption with high fat meal. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The volume of distribution is 3 to 3.5 L/kg and it does not cross the blood-brain barrier. Metabolism / Metabolites Primarily hepatic. Ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1 % of the administered dose excreted in the urine. Biological Half-Life Following oral administration, the half-life of ivermectin is approximately 18 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Single dose therapy with ivermectin has been associated with a low rate of serum aminotransferase elevations. A single case of clinically apparent liver injury has been reported after ivermectin use (Case 1). The onset of injury occurred 1 month after a single dose and was characterized by a hepatocellular pattern of serum enzyme elevations without jaundice. Recovery was rapid and complete. In trials of ivermectin to prevent SARS-CoV-2 infection and to ameliorate the course of early as well as severe COVID-19, serum aminotransferase elevations were not uncommon but were no more frequent among patients receiving ivermectin than among those receiving placebo or a comparator drug. Likelihood score: D (possible rare cause of mild clinically apparent liver injury). Protein Binding 93% |
| 参考文献 |
|
| 其他信息 |
LSM-5397 is a milbemycin.
Ivermectin is a semi-synthetic antiparasitic medication derived from avermectins, a class of highly-active broad-spectrum antiparasitic agents isolated from the fermentation products of Streptomyces avermitilis. Ivermectin itself is a mixture of two avermectins, comprising roughly 90% 5-O-demethyl-22,23-dihydroavermectin A1a (22,23-dihydroavermectin B1a) and 10% 5-O-demethyl-25-de(1-methylpropyl)-22,23-dihydro-25-(1-methylethyl)avermectin A1a (22,23-dihydroavermectin B1b). Ivermectin is mainly used in humans in the treatment of onchocerciasis, but may also be effective against other worm infestations (such as strongyloidiasis, ascariasis, trichuriasis and enterobiasis). Applied topically, it may be used in the treatment of head lice infestation. With the advent of 2020 and the COVID-19 pandemic, ivermectin began garnering notoriety due to its off-label use for the prophylaxis and treatment of COVID-19. While studies are still ongoing, much of the evidence for ivermectin in COVID-19 relies on pre-print in vitro data, and the clinical utility of this data remains unclear. Due to a number of factors - for example, the relatively low number of patients per trial and the speed at which these trials were conducted - studies on the use of ivermectin in COVID-19 have been fraught with statistical errors and accusations of plagiarism. In addition, the use of aggregate patient data in large-scale meta-analyses (as opposed to individual patient data (IPD)) has been shown to disguise otherwise blatant data errors, such as extreme terminal digit bias and the duplication of blocks of patient records. Until high-quality, peer-reviewed data regarding both the safety and efficacy of ivermectin for COVID-19 in humans becomes available, the use of ivermectin for these purposes should be avoided in favour of thoroughly-vetted therapies (e.g. COVID-19 vaccines like [Comirnaty](https://go.drugbank.com/drugs/DB15696)). Ivermectin is an antiinfective agent with activity against several parasitic nematodes and scabies and is the treatment of choice for onchocerciasis (river blindness). It is typically given as one or two oral doses. Ivermectin therapy has been associated with minor, self-limiting serum aminotransferase elevations and very rare instances of clinically apparent liver injury. Ivermectin B1a has been reported in Streptomyces avermitilis with data available. Ivermectin is an orally bioavailable macrocyclic lactone derived from Streptomyces avermitilis, with antiparasitic and potential anti-viral activities. Upon administration, ivermectin exerts its anthelmintic effect through binding and activating glutamate-gated chloride channels (GluCls) expressed on nematode neurons and pharyngeal muscle cells. This causes increased permeability of chloride ions, causing a state of hyperpolarization and results in the paralysis and death of the parasite. Ivermectin may exerts its antiviral effect, including its potential activity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), by binding to the importin (IMP) alpha/beta1 heterodimer, which is responsible for the nuclear import of viral proteins such as the integrase (IN) protein. This inhibits nuclear import of host and viral proteins and may inhibit viral replication. A mixture of mostly avermectin H2B1a (RN 71827-03-7) with some avermectin H2B1b (RN 70209-81-3), which are macrolides from STREPTOMYCES avermitilis. It binds glutamate-gated chloride channel to cause increased permeability and hyperpolarization of nerve and muscle cells. It also interacts with other CHLORIDE CHANNELS. It is a broad spectrum antiparasitic that is active against microfilariae of ONCHOCERCA VOLVULUS but not the adult form. See also: Ivermectin (annotation moved to). Drug Indication Administered topically, ivermectin cream is indicated for the treatment of inflammatory lesions associated with rosacea. An over-the-counter ivermection lotion is commercially available and indicated for the topical treatment of head lice infestations in patients ≥6 months of age. Orally administered ivermectin is indicated as a broad-spectrum anti-parasitic for the treatment of intestinal strongyloidiasis caused by _Strongyloides stercoralis_ and onchocerciasis caused by _Onchocerca volvulus_. Systemic ivermectin therapy is used internationally for the treatment of various tropical diseases, including filariasis, cutaneous larva migrans, and _Loa loa_ infection, amongst others. FDA Label Treatment of rosacea Mechanism of Action Ivermectin binds selectively and with high affinity to glutamate-gated chloride ion channels in invertebrate muscle and nerve cells of the microfilaria. This binding causes an increase in the permeability of the cell membrane to chloride ions and results in hyperpolarization of the cell, leading to paralysis and death of the parasite. Ivermectin also is believed to act as an agonist of the neurotransmitter gamma-aminobutyric acid (GABA), thereby disrupting GABA-mediated central nervous system (CNS) neurosynaptic transmission. Ivermectin may also impair normal intrauterine development of _O. volvulus_ microfilariae and may inhibit their release from the uteri of gravid female worms. |
| 分子式 |
C48H74O14
|
|---|---|
| 分子量 |
875.1
|
| 精确质量 |
874.508
|
| CAS号 |
70288-86-7
|
| PubChem CID |
6321424
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
155 °C
|
| LogP |
5.601
|
| tPSA |
170.06
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
62
|
| 分子复杂度/Complexity |
1680
|
| 定义原子立体中心数目 |
20
|
| SMILES |
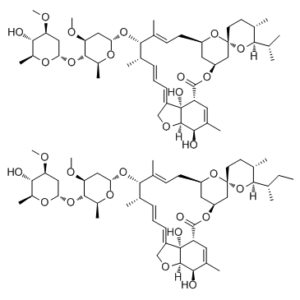
CC[C@H](C)[C@@H]1[C@H](CC[C@@]2(O1)C[C@@H]3C[C@H](O2)C/C=C(/[C@H]([C@H](/C=C/C=C/4\CO[C@H]5[C@@]4([C@@H](C=C([C@H]5O)C)C(=O)O3)O)C)O[C@H]6C[C@@H]([C@H]([C@@H](O6)C)O[C@H]7C[C@@H]([C@H]([C@@H](O7)C)O)OC)OC)\C)C
|
| InChi Key |
AZSNMRSAGSSBNP-ZGXOMDHGSA-N
|
| InChi Code |
InChI=1S/C48H74O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38/h12-15,19,25-26,28,30-31,33-45,49-50,52H,11,16-18,20-24H2,1-10H3/b13-12+,27-15+,32-14+/t25-,26+,28+,30+,31+,33-,34+,35+,36+,37+,38+,39+,40-,41+,42+,43-,44+,45-,47-,48-/m1/s1
|
| 化学名 |
(1R,4S,5'S,6R,6'R,8R,10E,12S,13S,14E,16E,20R,21R,24S)-6'-[(2R)-butan-2-yl]-21,24-dihydroxy-12-[(2R,4S,5S,6S)-5-[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-4-methoxy-6-methyloxan-2-yl]oxy-5',11,13,22-tetramethylspiro[3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6,2'-oxane]-2-one
|
| 别名 |
MK-933; L-64047; MK 933; L64047; MK-0933; Noromectin; MK 933; Mectizan; MK 0933; Ivermectin; Ivomec; L 64047; Pandex.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100~250 mg/mL ( 114.27~285.68 mM )
Ethanol : ~100 mg/mL H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.86 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.38 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (2.86 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1427 mL | 5.7136 mL | 11.4273 mL | |
| 5 mM | 0.2285 mL | 1.1427 mL | 2.2855 mL | |
| 10 mM | 0.1143 mL | 0.5714 mL | 1.1427 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|
|
|
|
|