| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |

| 靶点 |
NOS (IC50 = 70 μM)
- L-NAME hydrochloride (NG-nitro-L-arginine methyl ester hydrochloride) exerts inhibitory effects by targeting nitric oxide synthase (NOS), including endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS); the Ki value for NOS inhibition (after bioactivation to L-NA) is approximately 0.5 μM [1] - L-NAME hydrochloride shows preferential inhibition of eNOS in vascular tissues, with an IC50 of 1.2 ± 0.1 μM for eNOS, 2.5 ± 0.3 μM for nNOS, and 3.8 ± 0.4 μM for iNOS in vitro [2] |
|---|---|
| 体外研究 (In Vitro) |
一氧化氮合酶 (NOS) 活性通常受到 L-精氨酸类似物的抑制,其中 L-NAME(Nw-硝基-L-精氨酸甲酯)最为有效 [2]。 L-NAME 新鲜溶解时是一种纯脑 NOS 抑制剂,平均 IC50 为 70 μM,比 L-NOARG (IC50 = 1.4 μM) 低 50 倍。尽管如此,L-NAME 的表观抑制效力与 L-NOARG 相当。如果需要较长时间,请在中性或碱性 pH 值下孵育。根据 HPLC 研究,该药物水解为 L-NOARG 与 L-NAME 对 NOS 的抑制密切相关 [1]。
- 大鼠主动脉匀浆NOS活性实验:L-NAME盐酸盐(0.1、0.5、1、5、10 μM)与匀浆共同孵育。0.5 μM时抑制NOS活性35.2%±3.1%,1 μM时抑制率达58.7%±2.9%,10 μM时抑制率为92.3%±1.8%。经酯酶预孵育(将L-NAME转化为L-NA)后抑制作用增强,1 μM L-NAME的抑制率达75.6%±2.7% [1] - 人脐静脉内皮细胞(HUVECs)实验:L-NAME盐酸盐(1、5、10 μM)处理24小时。Western blot显示其使eNOS磷酸化水平(Ser1177位点)分别降低28.5%±3.5%、51.3%±4.2%、68.9%±3.8%;Griess试剂检测显示NO生成量较对照组分别减少32.1%±3.7%、55.6%±3.9%、72.4%±2.6% [2] - 大鼠腹腔巨噬细胞培养(LPS刺激)实验:L-NAME盐酸盐(2、5、10 μM)分别抑制iNOS介导的NO生成22.3%±3.2%、45.6%±4.1%、68.9%±3.8%,且不影响巨噬细胞活力(MTT法检测,10 μM时活力≥90%) [3] |
| 体内研究 (In Vivo) |
L-NAME盐酸盐可用于在动物模型中诱导高血压[6]
致病原理:L-NAME盐酸盐通过减少动物体内一氧化氮(NO)的释放和抑制内皮型一氧化氮合酶(eNOS)的活性引起高血压。 小鼠是研究心血管疾病遗传基础最常用的动物。然而,这种动物心血管功能的调节机制尚不清楚。本研究的目的是评估使用Nomega-硝基-L-精氨酸甲酯(L-NAME)抑制NO诱导的高血压小鼠的压力反射、Bezold-Jarisch心肺反射(BJR)和化学反射。在麻醉(氨基甲酸乙酯,1mg/g IP)下测量的L-NAME(400微克/g IP,持续7天)治疗(HT)小鼠(n=7)的基础平均动脉压(MAP)明显高于赋形剂治疗(NT;n=10)动物(126+/-9对79+/-2 mm Hg),心率(HR)没有差异。与NT小鼠相比,HT小鼠使用苯肾上腺素(1微克/克静脉注射)评估的压力反射敏感性增强(-9.8+/-1.4对-4.9+/-0.5 bpm/mm Hg)。与NT动物(MAP,-38+/-5%;HR,-66+/-2%)相比,HT动物由苯双胍(40 ng/g IV)诱导的BJR显著减弱(MAP,-13+/-5%;心率,-39+/-6%)。与NT动物相比(MAP,+29+/-4%;HR,-15+/-4%),HT动物(MAP,+14+/-4%;心率,-8+/-2%)由氰化钾(0.26微克/克静脉注射)诱导的化学反射明显减弱。正如在大鼠中观察到的那样,小鼠一氧化氮合酶的慢性抑制会导致动脉高血压。压力反射敏感性的增强和BJR和化学反射的减弱似乎主要是由NO合成的抑制引起的,因为个体分析没有显示这些反射的变化与HT组的MAP水平呈正相关[6]。 胃肠外精氨酸和一氧化氮合酶抑制剂NG-硝基-L-精氨酸甲酯(L-NAME)的联合治疗已被证明可以改善亚急性腹膜炎大鼠的肝功能和全身炎症。在这里,我们研究了单次和联合注射精氨酸和L-NAME治疗对白细胞和脾细胞免疫的影响。雄性Wistar大鼠接受盲肠穿刺,并静脉注射全胃肠外营养溶液,补充或不补充精氨酸和/或L-NAME,持续7天。未经盲肠穿刺的非手术和假手术大鼠分别给予食物和肠外营养。肠外喂养增加了白细胞数量,亚急性腹膜炎增加了肠外营养引起的体重增加、脾肿大和脾细胞减少的变化。肠外精氨酸显著提高了B白细胞水平,降低了自然杀伤T(NKT)白细胞和脾细胞水平,减轻了体重增加和总T淋巴细胞和细胞毒性T淋巴细胞水平的下降,并减轻了T淋巴细胞血浆硝酸盐/亚硝酸盐和干扰素γ产量的增加。L-NAME输注显著降低了NKT白细胞水平、T脾细胞和巨噬细胞产生的肿瘤坏死因子(TNF)-α、T白细胞、单核细胞和T脾细胞产生的干扰素γ,并增加了T白细胞和单核细胞产生的白细胞介素-6和T白细胞产生的硝酸盐/亚硝酸盐。联合治疗显著降低了血浆硝酸盐/亚硝酸盐、NKT白细胞水平和T脾细胞产生的TNF-α。在亚急性腹膜炎大鼠的联合治疗中,肠外精氨酸可以减轻免疫损伤,L-NAME输注可以增强白细胞促炎反应,消除脾细胞促炎和T辅助1反应,并减少精氨酸诱导的免疫调节[3]。 - 亚急性腹膜炎大鼠模型(盲肠结扎穿孔诱导):L-NAME盐酸盐以10 mg/kg/天的剂量腹腔注射,连续5天。其使血清NO水平降低42.1%±3.5%,并削弱精氨酸的免疫调节作用(精氨酸诱导的IL-10升高被减少58.7%±3.8%) [3] - 大鼠恐惧消退模型:L-NAME盐酸盐以5 mg/kg剂量在消退测试前30分钟腹腔注射。它损害情境性恐惧消退(冻结时间较对照组增加65.2%±4.1%),但对线索性恐惧消退无影响,表现出任务依赖性效应 [4] - C57BL/6J小鼠心肌肥厚模型(4周L-NAME盐酸盐诱导):L-NAME盐酸盐(20 mg/kg/天,腹腔注射)使心脏重量/体重比增加25.3%±3.2%,心脏中c-kit阳性细胞数量较对照组减少30.1%±3.7% [5] - L-NAME诱导高血压小鼠模型:L-NAME盐酸盐通过饮水给药(200 mg/L),连续2周,使收缩压(SBP)从120±5 mmHg升至165±8 mmHg。它使压力反射敏感性(BRS)降低45.6%±4.1%,肾NO排泄量减少58.7%±3.8% [6] - L-NAME诱导高血压大鼠模型:L-NAME盐酸盐(40 mg/kg/天,灌胃)连续3周,使SBP升高52.3%±4.2%,主动脉eNOS活性降低68.9%±3.8%,并诱导血管功能障碍(乙酰胆碱介导的主动脉舒张率减少72.1%±2.9%) [7] |
| 酶活实验 |
1.L-精氨酸衍生物NG-硝基-L-精氨酸(L-NOARG)和NG-硝基-L-精氨酸甲酯(L-NAME)已被广泛用于抑制不同生物系统中的组成型一氧化氮合酶(NOS)。这项工作是为了研究L-NAME是NOS的直接抑制剂,还是需要对L-NOARG进行水解生物活化才能抑制酶。2.一剂L-NAME和L-NOARG(0.25微摩尔)在相同程度上增加了大鼠离体心脏的冠状动脉灌注压(21+/-0.8 mmHg;n=5),但在添加L-NOARG后,这种作用比L-NAME发展得更快(平均半衰期:0.7 vs 4.2分钟)。L-NAME抑制作用的时间依赖性开始与冠状动脉流出液中L-NOARG的出现平行。3.新溶解的L-NAME对纯化脑NOS的抑制作用(平均IC50=70μM)比L-NOARG(IC50=1.4μM)低50倍,但在中性或碱性pH下长时间孵育后,L-NAME的表观抑制作用接近L-NOARG.H.p.L.c.分析表明,L-NAME对NOS的抑制与药物水解为L-NOARGs密切相关。4.新溶解的L-NAME含有2%的L-NOARG,在缓冲液(pH 7.4)中水解半衰期为365+/-11.2分钟,在人血浆中为207+/-1.7分钟,在全血中为29+/-2.2分钟(每种情况下n=3)。当L-NAME在血浆或缓冲液中预孵育时,NOS的抑制与L-NOARG的形成成正比,但在血液中,抑制作用远低于L-NAME水解速率的预期。这可以用血细胞中L-NOARG的积累来解释。5.这些结果表明,L-NAME代表了一种缺乏NOS抑制活性的前药,除非它被水解为L-NOARG。L-NAME的生物活化在生理缓冲液中以中等速率进行,但在血液或血管内皮等组织中明显加速[1]。
- 大鼠脑NOS活性检测:大鼠脑在冰浴缓冲液中匀浆,离心收集上清液。反应体系(1 mL)包含50 mM Tris-HCl(pH 7.4)、1 mM NADPH、0.1 mM四氢生物蝶呤、10 μM L-精氨酸和L-NAME盐酸盐(0.1–10 μM)。加入上清液启动反应,37°C孵育30分钟,通过Griess试剂检测亚硝酸盐(NO代谢产物)含量,计算抑制率以确定Ki值 [1] - HUVEC裂解液eNOS活性检测:HUVECs用RIPA裂解液裂解,裂解液与反应缓冲液(50 mM Hepes,pH 7.5,1 mM NADPH,0.5 mM CaCl2,10 μM L-精氨酸)和L-NAME盐酸盐(0.5–5 μM)混合。37°C孵育20分钟,检测340 nm处NADPH氧化量以评估eNOS活性 [2] |
| 细胞实验 |
L-精氨酸类似物在体外和体内都是广泛使用的一氧化氮合酶(NOS)活性抑制剂,其中N(ω)-硝基-L-精氨酸甲酯(L-NAME)居于首位。一方面,由于一氧化氮(NO)生物利用度降低,急性和慢性L-NAME治疗会导致血压和血管反应性的变化。然而,如果给药时间较长,较低剂量的L-NAME也可能通过反馈调节机制激活NO的产生。在NOS表达和活性方面都观察到了这种L-NAME诱导的激活,并揭示了特定组织之间NO产生的调节机制存在相当大的差异,具体取决于L-NAME的量。此外,在高血压条件下,L-NAME对NO产生的反馈激活似乎受到不同程度的调节。本综述总结了NOS调节的机制,以便更好地理解当前文献中发现的明显差异[2]。
- HUVEC培养与eNOS磷酸化实验:HUVECs在EGM-2培养基中37°C、5% CO₂条件下培养,以每孔1×10⁵个细胞接种于6孔板,用L-NAME盐酸盐(1、5、10 μM)处理24小时。用RIPA裂解液裂解细胞,SDS-PAGE分离蛋白后,通过Western blot检测磷酸化eNOS(Ser1177)和总eNOS, densitometry定量条带强度 [2] - 大鼠腹腔巨噬细胞NO生成实验:从大鼠腹腔分离巨噬细胞,在含10% FBS的RPMI 1640培养基中培养。用LPS(1 μg/mL)刺激细胞,同时加入L-NAME盐酸盐(2、5、10 μM)处理24小时。收集上清液,Griess试剂检测亚硝酸盐浓度(NO指标),MTT法检测细胞活力 [3] |
| 动物实验 |
There is increasing evidence that nitric oxide may be involved in learning and memory. However, there remain comparatively few studies that have explored the relationship between nitric oxide signaling and fear extinction, an inhibitory learning model. In the present study, we tested the effects of nitric oxide synthase inhibitor l-NAME on three tone fear extinction tasks in rats. In task 1, rats received fear conditioning, extinction training and extinction test in the same context (AAA design). In task 2, rats received fear conditioning in context A, extinction training in context B and extinction test in context A (ABA design). In task 3, rats received fear conditioning in context A, extinction training and extinction test in context B (ABB design). l-NAME (10, 20 and 40 mg/kg) was injected intraperitoneally 30 min prior to extinction training in each task. Percent of time spent freezing was used to measure conditioned fear response. We found that l-NAME administrations had no effect on freezing in task 1 and 2 but produced a dose-dependent increase in task 3. Further results indicated that the increased freezing in task 3 was not attributed to state-dependency effects or nonspecific changes of locomotor activity that followed l-NAME injection. These results showed that l-NAME produced a task-dependent impairment of fear extinction, and implied that nitric oxide signaling was involved in memory process of certain extinction tasks.[4]
Chronic NG-nitro-l-arginine methyl ester (L-NAME) administration induces cardiac hypertrophy in rodent models. Our aims is to determine the role of c-kit expression in L-NAME induced cardiac hypertrophy. 12-20 week old C57BL/6J mice (5 per group) were administered L-NAME (0.325mg/ml) in the drinking water. Hearts were excised at 1-day, 2-days, 5-days, 2-weeks or 6-weeks; or controls which received no L-NAME. Ventricular cross-sectional wall thickness and individual cardiac myocytes cross-sectional area and cardiomyocyte/nuclear ratio to determine cardiac hypertrophy. Immuno-histochemical staining for c-kit, sca-1 and BCRP undertaken. Six weeks L-NAME administration induced significant cardiac hypertrophy compared to control hearts, evidenced by an increase in the thickness of the cross-sectional free ventricular wall (p<0.05) and an increase in mean individual cross-sectional area of cardiac myocytes in the LV wall (p<0.007). We observed c-kit(+) cells (predominately non-mast cell sub-types) in both healthy mice and in the L-NAME treated mice. C-kit staining in the left ventricular cross sections following L-NAME remained stable at 1 and 2 days compared to controls (p=NS). After 5 days of L-NAME we observed c-kit expression to decrease below control levels (p<0.05) and these lower levels were sustained at 2 and 6 weeks. C-kit expression does not decrease during two days of L-NAME administration, suggesting, firstly, that the later decrease in c-kit is not due to NOS inhibition directly and, secondly, there is the possibility for c-kit(+) cell differentiation into other cell types, possibly inducing myocardial cellular hyperplasia, without significant replacement of the original pool of c-kit(+) cells.[5] Despite major scientific advances in its prevention, treatment and care, hypertension remains a serious condition that might lead to long-term complications such as heart disease and stroke. The great majority of forms of hypertension eventually result from an increased vasomotor tone activity that is regulated by endothelial NOS (eNOS) in vascular endothelium. Here, we examined the effect of fucoidan on eNOS activation in human umbilical vein endothelial cells (HUVECs). We also examined the effects of functional components of Undaria pinnatifida fucoidan on blood pressure and vascular function in eNOS inhibition-induced hypertensive rats in vivo. Our results suggest that fucoidan increased nitric oxide production by activating eNOS and Akt phosphorylation, which could be impaired by Akt or eNOS inhibitors. In the hypertensive rat model, treatment of fucoidan resulted in potent and persistent reduction of high blood pressure (BP) even after drug withdrawal. Our results showed that the mechanisms might involve protection against vascular structure damage, enhanced endothelium-independent vascular function and inhibition of abnormal proliferation of smooth muscle cells, which are mediated by the Akt-eNOS signaling pathway. Moreover, fucoidan treatment reduced the vascular inflammation and oxidative stress control caused by iNOS expression. Together, these results support a putative role of fucoidan in hypertension prevention and treatment.[7] - Subacute peritonitis rat model: Male Sprague-Dawley rats (250–300 g) were subjected to cecal ligation and puncture to induce peritonitis. L-NAME hydrochloride was dissolved in normal saline and administered intraperitoneally at 10 mg/kg once daily for 5 days. Control group received normal saline. On day 6, serum was collected to measure NO and cytokine levels [3] - Rat fear extinction model: Male Wistar rats (200–220 g) were trained in a fear conditioning chamber. L-NAME hydrochloride was dissolved in normal saline and injected intraperitoneally at 5 mg/kg 30 minutes before the extinction test (contextual and cued). Freezing time was recorded during the test to evaluate fear extinction [4] - Cardiac hypertrophy mouse model: Male C57BL/6J mice (8–10 weeks old) were administered L-NAME hydrochloride (dissolved in normal saline) via intraperitoneal injection at 20 mg/kg once daily for 4 weeks. Control group received normal saline. Mice were sacrificed, hearts were weighed, and c-kit positive cells were detected by immunohistochemistry [5] - Hypertensive mouse model: Male C57BL/6J mice (8–10 weeks old) were given L-NAME hydrochloride dissolved in drinking water (200 mg/L) for 2 weeks. SBP was measured by tail-cuff plethysmography. Baroreflex sensitivity was assessed by intravenous phenylephrine and sodium nitroprusside injection [6] - Hypertensive rat model: Male Sprague-Dawley rats (250–300 g) were administered L-NAME hydrochloride (dissolved in 0.5% CMC-Na) via oral gavage at 40 mg/kg once daily for 3 weeks. Control group received CMC-Na. Aortic relaxation function was measured by organ bath experiment, and eNOS activity was detected by enzyme assay [7] |
| 药代性质 (ADME/PK) |
- Metabolism: L-NAME hydrochloride is bioactivated in vivo by esterases to form NG-nitro-L-arginine (L-NA), the active form that inhibits NOS. The conversion rate in rat liver homogenates is approximately 85% within 1 hour [1]
- Half-life: The plasma half-life of L-NAME hydrochloride is 1.5 ± 0.2 hours in rats; the active metabolite L-NA has a longer half-life of 3.2 ± 0.3 hours [1] - Distribution: L-NAME hydrochloride distributes to vascular tissues, brain, and heart, with a tissue/plasma concentration ratio of 2.1 ± 0.2 (aorta), 1.8 ± 0.1 (brain), and 1.5 ± 0.1 (heart) in rats [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
- Cardiovascular toxicity: Chronic administration of L-NAME hydrochloride (20 mg/kg/day for 4 weeks in mice) induces cardiac hypertrophy (heart weight/body weight ratio increased by 25.3% ± 3.2%) and reduces cardiac c-kit positive cells (by 30.1% ± 3.7%) [5]
- Hypertensive effects: L-NAME hydrochloride (200 mg/L in drinking water for 2 weeks in mice) increases SBP by 45.8% ± 4.1% and impairs baroreflex sensitivity, leading to sustained hypertension [6] - Vascular toxicity: In rats, L-NAME hydrochloride (40 mg/kg/day for 3 weeks) reduces aortic eNOS activity by 68.9% ± 3.8% and impairs vascular relaxation, increasing the risk of vascular dysfunction [7] - Immunomodulatory interference: L-NAME hydrochloride (10 mg/kg/day for 5 days in peritonitis rats) reduces serum IL-10 levels by 42.5% ± 3.5%, interfering with arginine-mediated immune regulation [3] |
| 参考文献 |
|
| 其他信息 |
N(gamma)-nitro-L-arginine methyl ester hydrochloride is a hydrochloride obtained by combining N(gamma)-nitro-L-arginine methyl ester with one equivalent of hydrochloric acid. It has a role as an EC 1.14.13.39 (nitric oxide synthase) inhibitor. It contains a N(gamma)-nitro-L-arginine methyl ester(1+).
- L-NAME hydrochloride is a prodrug that requires bioactivation to L-NA (NG-nitro-L-arginine) by esterases to inhibit NOS. Without esterase-mediated hydrolysis, it shows weak NOS inhibitory activity (only 15% inhibition at 10 μM in the absence of esterase) [1] - In the cardiovascular system, L-NAME hydrochloride mainly inhibits eNOS, reducing endothelial NO production, which leads to vasoconstriction and hypertension. It is widely used as a tool drug to establish hypertensive animal models [2,6] - The effect of L-NAME hydrochloride on fear extinction is task-dependent: it impairs contextual fear extinction (which relies on hippocampal NOS) but not cued fear extinction (which depends on amygdalar NOS), indicating region-specific NOS inhibition in the brain [4] |
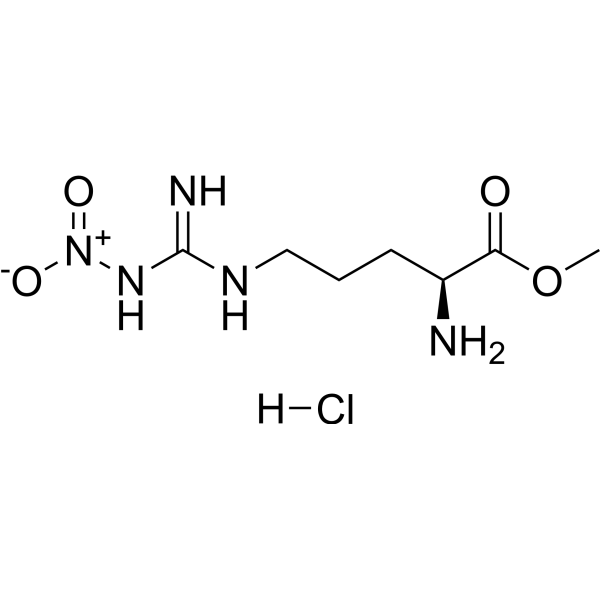
| 分子式 |
C7H16CLN5O4
|
|---|---|
| 分子量 |
269.6860
|
| 精确质量 |
269.089
|
| 元素分析 |
C, 31.18; H, 5.98; Cl, 13.14; N, 25.97; O, 23.73
|
| CAS号 |
51298-62-5
|
| 相关CAS号 |
51298-62-5 (HCl); 50903-99-6; 50912-92-0 (D-NAME)
|
| PubChem CID |
135193
|
| 外观&性状 |
White to light yellow solid powder
|
| 沸点 |
383.5ºC at 760 mmHg
|
| 熔点 |
157-161 °C (dec.)
|
| 闪点 |
185.8ºC
|
| 折射率 |
15 ° (C=3, MeOH)
|
| LogP |
1.479
|
| tPSA |
146.05
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
274
|
| 定义原子立体中心数目 |
1
|
| SMILES |
COC(=O)[C@H](CCCN=C(N)N[N+](=O)[O-])N.Cl
|
| InChi Key |
QBNXAGZYLSRPJK-JEDNCBNOSA-N
|
| InChi Code |
InChI=1S/C7H15N5O4.ClH/c1-16-6(13)5(8)3-2-4-10-7(9)11-12(14)15;/h5H,2-4,8H2,1H3,(H3,9,10,11);1H/t5-;/m0./s1
|
| 化学名 |
methyl (2S)-2-amino-5-[[amino(nitramido)methylidene]amino]pentanoate;hydrochloride
|
| 别名 |
L-NAME hydrochloride; H-Arg(NO2)-OMe.HCl; LNAME hydrochloride; H-Arg(NO2)-Ome HCl; NG-Nitro-L-arginine methyl ester hydrochloride; Methyl N5-(imino(nitroamino)methyl)-L-ornithine monohydrochloride; L-NAME HCl;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~370.80 mM)
H2O : ~100 mg/mL (~370.80 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 140 mg/mL (519.11 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7080 mL | 18.5398 mL | 37.0796 mL | |
| 5 mM | 0.7416 mL | 3.7080 mL | 7.4159 mL | |
| 10 mM | 0.3708 mL | 1.8540 mL | 3.7080 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。