| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
α1-adrenergic receptor; β-adrenoceptor
|
|---|---|
| 体外研究 (In Vitro) |
拉贝洛尔对豚鼠心脏和肺膜上的 β- 首先素能位点表现出更大的亲和力 (IC50 分别为 0.8 和 4.0 μM)[2]。 拉贝洛尔对兔子宫膜上的 α- 首先素能结合位Labctalol对心脏膜中β结合位点的结合亲和力是心肌膜中α结合位点的19倍[2](IC50=15 μM)。
|
| 体内研究 (In Vivo) |
拉贝洛尔 (10 mg/kg;ih) 通过血脑屏障,注射后 90 分钟在 10 日龄幼鼠大脑中达到 2.1 ug/g 组织水平[4]。拉贝洛尔 (5.0 mg/kg;ip) 部分尾震应激串联中的循环IL-1β和IL-6[5]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
100mg and 200mg oral doses of labetalol have a Tmax of 20 minutes to 2 hours. Bioavailability may be as low as 11% or as high as 86% and may increase in older patients or when taken with food. Radiolabelled doses of labetalol are 55-60% recovered in the urine and 12-27% recovered in the feces. In normotensive patients, the volume of distribution is 805L. In hypertensive patients, the volume of distribution is between 188-747L with an average of 392L. Labetalol has a plasma clearance of approximately 1500mL/min and a whole blood clearance of 1100mL/min. Cardioselective beta-blockers /including labetalol/ tend to be hydrophilic, whereas nonselective beta blockers tend to be lipophilic. The property of lipophilicity is an essential factor for the expression of nonselectivity. Lopophilic beta-blockers generally undergo hepatic elimination and distribute well into all body compartments, including the brain. Hydrophilic beta-blockers usually are excreted unchanged by the kidney and enter deep body compartments with difficulty. /From table/ Beta-blocker drugs are rapidly absorbed, with a bioavailability varying between 30% and 90% because of the large first-pass hepatic extraction. ... Most beta-blockers ... have apparent volumes of distribution greater than 1 l/kg /Class II beta-blockers/ Labetalol hydrochloride is rapidly and approximately 90-100% absorbed from the GI tract following oral administration, but the drug undergoes extensive first pass metabolism in the liver and/or GI mucosa. Only about 25% of an oral dose reaches systemic circulation unchanged in fasted adults. Although absolute bioavailability in one study reportedly ranged from 11-86% (mean: 33%) following oral administration of a single 100 mg dose in fasted adults, the considerable interindividual variability in this study may have resulted from use of a relatively insensitive spectrofluorometric assay. Food delays GI absorption of labetalol hydrochloride but increases absolute bioavailability of the drug, possibly by decreasing first pass metabolism and/or hepatic blood flow. Following oral administration of a single 200 mg dose in healthy adults in one study, absolute bioavailability of the drug averaged 26 and 36% in the fasted and nonfasted state, respectively. First pass metabolism may also be reduced and bioavailability substantially increased in geriatric patients and in patients with hepatic dysfunction. However, in one study in patients with hepatosplenic schistosomiasis, mean absolute bioavailability of the drug was reportedly decreased when compared with healthy individuals. Oral cimetidine increases, and glutethimide decreases, the bioavailability of labetalol. Concomitant oral administration of labetalol hydrochloride and hydrochlorothiazide does not affect the bioavailability of either drug. Following multiple dose oral administration of labetalol hydrochloride, peak plasma concentrations are generally achieved within 40 min to 2 hr. Peak plasma concentrations reportedly increase proportionately with oral dosage at dosages ranging from 100 mg to 3 g daily. In one study in hypertensive patients, peak plasma labetalol concentration following oral administration of 200 mg 3 times daily or 300 mg twice daily averaged 323 or 430 ng/ml, respectively, and the steady state plasma drug concentration averaged 149 or 145 ng/ml, respectively; based on pharmacokinetic and pharmacodynamic (ie, blood pressure response) evaluation, these dosage regimens were considered equivalent. Following iv injection over 1 min of a 1.5 mg/kg dose of labetalol hydrochloride in one study, a mean peak plasma concentration of about 5.7 ug/ml occurred 2 min after injection and plasma concentration had declined to an average of 575 ng/ml at 10.5 min after injection. For more Absorption, Distribution and Excretion (Complete) data for LABETALOL (14 total), please visit the HSDB record page. Metabolism / Metabolites The metabolism of labetalol has not been fully described in the literature but studies in sheep show an N-dealkylation to 3-amino-1-phenyl butane. This metabolite may be further metabolized to benzylacetone and 3-amino-(4-hydroxyphenyl)butane. Labetalol in humans is mainly metabolized to glucuronide metabolites such as the O-phenyl-glucuronide and the N-glucuronide. Labetalol is extensively metabolized in the liver and possibly in the GI mucosa following oral administration, principally by conjugation with glucuronic acid. The major metabolite is the O-alkylglucuronide, with smaller amounts of the O-phenylglucuronide and N-glucuronide being formed. Following oral administration, labetalol undergoes extensive first pass metabolism in the liver and/or GI mucosa. Primarily hepatic, undergoes significant first pass metabolism Route of Elimination: These metabolites are present in plasma and are excreted in the urine, and via the bile, into the feces. Half Life: 6-8 hours Biological Half-Life Labetalol has a half life of 1.7-6.1 hours. Plasma concentrations of labetalol appear to decline in a biphasic or possibly triphasic manner. In healthy adults and adults with hypertension, the half-life in the distribution phase has been reported to average 6-44 min and the half-life in the terminal elimination phase (t1/2beta) has been reported to average 2.5-8 hr. The variability in reported mean half-lives for the drug may have resulted in part from use of a relatively insensitive spectrofluorometric assay in some studies. The manufacturers state that the drug has a plasma elimination half-life of 5.5 or 6-8 hr following iv or oral administration, respectively. The elimination half-life of the drug appears to be unchanged in individuals with renal or hepatic impairment, but may be increased in patients with severe renal impairment (eg; creatinine clearance less than 10 ml/min) undergoing dialysis and slightly increased (but within the reported range) in geriatric individuals. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Labetalol has two asymmetric centers and therefore, exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R' stereoisomer, makes up 25% of racemic labetalol. Labetalol HCl combines both selective, competitive, alpha-1-adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha- to beta- blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous (IV) administration, respectively. Beta-2-agonist activity has been demonstrated in animals with minimal beta-1-agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta- adrenergic blockade, a membrane stabilizing effect has been demonstrated. Toxicity Data LD50 = 66 mg/kg (Rat, parental-intravenous) Interactions Reproduction studies in rats or rabbits using combined oral labetalol hydrochloride and hydrochlorothiazide dosages up to about 15 and 80 times the maximum recommended human dosage, respectively, have not revealed evidence of teratogenicity, although combined oral dosages 3.5 and 20 times the maximum recommended human dosage, respectively, were maternotoxic with resultant fetotoxicity in rabbits. The combination appeared to be more toxic than either drug alone in rabbits. /Labetalol hydrochloride/ When labetalol is administered with diuretics or other hypotensive drugs, the hypotensive effect may be increased. ... When beta-adrenergic blocking agents are administered with calcium-channel blocking agents, therapeutic as well as adverse effects may be additive. Concomitant administration of iv labetalol and halothane anesthesia results in asynergistic hypotensive effect, the degree and duration of which may be controlled by adjusting the halothane concentration; however, excessive hypotension can result in a large reduction in cardiac output and an increase in central venous pressure. Concomitant administration of oral cimetidine has been shown to substantially increase the absolute bioavailability of oral labetalol, possibly via enhanced absorption or decreased first pass hepatic metabolism of labetalol. For more Interactions (Complete) data for LABETALOL (10 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse oral approx 0.6 g/kg /Labetolol hydrochloride/ LD50 Rat oral > 2 g/kg /Labetalol hydrochloride/ LD50 Dog oral > 1 g/kg /Labetolol hydrochloride/ |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Adrenergic alpha-Antagonists; Adrenergic beta-Antagonists; Antihypertensive Agents; Sympatholytics Antihypertensive Labetalol hydrochloride is an alpha and beta-adrenergic blocking agent. The drug is commercially available as a racemic mixture of its 4 stereoisomers. The RR isomer has about 2-4 times the beta-adrenergic blocking activity of the racemic mixture but has only minimal alpha1-adrenergic blocking activity; most of the alpha1-adrenergic blocking activity of the drug is attributable to the SR isomer. The RR isomer also appears to possess some beta2-agonist activity. /Labetalol hydrochloride/ Labetalol hydrochloride is used in the management of hypertension. The drug has been used as monotherapy or in combination with other classes of antihypertensive agents. The drug is at least as effective as pure beta-adrenergic blocking agents, thiazide diuretics, methyldopa, or clonidine. ... Iv labetalol hydrochloride is used to control blood pressure in patients with severe hypertension or hypertensive emergencies. Unlike other currently available parenteral hypotensive agents, labetalol usually produces a prompt, but gradual reduction of blood pressure without substantial changes in heart rate or cardiac output. Iv labetalol appears to adequately reduce blood pressure in about 80-90% of patients with severe hypertension or hypertensive emergencies, irrespective of etiology, and may be useful even when other drugs have failed. /Labetalol hydrochloride/ For more Therapeutic Uses (Complete) data for LABETALOL (9 total), please visit the HSDB record page. Drug Warnings Serious clinical effects of overdose involve primarily the cardiovascular system (bradycardia. hypotension, cardiogenic shock, pulmonary edema) and the central nervous system (coma, convulsions, apneal). In severe overdose, apnea and hemodynamic compromise may appear suddenly after ingestions of large beta-blocker doses. /Class II beta-blockers/ Adverse reations depend more on drug affinity for B1 and B2 receptors than overdose does. B1 Blockade (antagonist) activity causes decreased sinus rate, contractility, and conduction, decreased renin release, and decreased aqueous humor formation. B2 Blockade produces bronchiolar and arteriolar smooth muscle constriction, decreasd insulin secretion and decreased lipolysis and glucogenolysis resulting in decreased blood fatty acids and glucose. /Class II beta-Blockers/ Labetalol shares the toxic potentials of beta-adrenergic and postsynaptic alpha1-adrenergic blocking agents, and the usual precautions of these agents should be observed. When labetalol is used in fixed combination with hydrochlorothiazide, the cautions, precautions, and contraindications associated with thiazide diuretics must be considered in addition to those associated with labetalol. Labetalol should be used with caution in patients with inadequate cardiac function, since congestive heart failure may be precipitated by blockade of beta-adrenergic stimulation when labetalol therapy is administered. In addition, in patients with latent cardiac insufficiency, prolonged beta-adrenergic blockade may lead to cardiac failure. Although beta-adrenergic blocking agents should be avoided in patients with overt congestive heart failure, labetalol may be administered cautiously, if necessary, to patients with well-compensated heart failure (e.g., those controlled with cardiac glycosides and/or diuretics). Patients receiving labetalol therapy should be instructed to consult their physician at the first sign or symptom of impending cardiac failure and should be adequately treated (e.g., with a cardiac glycoside and/or diuretic) and observed closely; if cardiac failure continues, labetalol should be discontinued, gradually if possible. For more Drug Warnings (Complete) data for LABETALOL (17 total), please visit the HSDB record page. Pharmacodynamics Labetalol antagonizes various adrenergic receptors to decrease blood pressure. The duration of action is long as it is generally given twice daily, and the therapeutic window is wide as patients usually take 200-400mg twice daily. Patients susceptible to bronchospasms should not use labetalol unless they are unresponsive to or intolerant of other antihypertensives. |
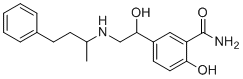
| 分子式 |
C19H24N2O3
|
|---|---|
| 分子量 |
328.4055
|
| 精确质量 |
328.178
|
| 元素分析 |
C, 69.49; H, 7.37; N, 8.53; O, 14.61
|
| CAS号 |
36894-69-6
|
| 相关CAS号 |
Labetalol hydrochloride; 32780-64-6
|
| PubChem CID |
3869
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
552.7±50.0 °C at 760 mmHg
|
| 闪点 |
288.1±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.609
|
| LogP |
2.31
|
| tPSA |
95.58
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
385
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O([H])C([H])(C1C([H])=C([H])C(=C(C(N([H])[H])=O)C=1[H])O[H])C([H])([H])N([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H]
|
| InChi Key |
SGUAFYQXFOLMHL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H24N2O3/c1-13(7-8-14-5-3-2-4-6-14)21-12-18(23)15-9-10-17(22)16(11-15)19(20)24/h2-6,9-11,13,18,21-23H,7-8,12H2,1H3,(H2,20,24)
|
| 化学名 |
2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl]benzamide
|
| 别名 |
Trandate; Labetalol; Normodyne; Apo-Labetalol; Albetol; Dilevalol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~125 mg/mL (~380.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (6.33 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.33 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0450 mL | 15.2249 mL | 30.4497 mL | |
| 5 mM | 0.6090 mL | 3.0450 mL | 6.0899 mL | |
| 10 mM | 0.3045 mL | 1.5225 mL | 3.0450 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05551104 | Recruiting | Drug: Oral Nifedipine Drug: Oral Labetalol |
Postpartum Complication Maternal Hypertension |
Loma Linda University | December 2023 | Not Applicable |
| NCT04298034 | Recruiting | Drug: Labetalol, Nifedipine | Preeclampsia Hypertension in Pregnancy |
Medical College of Wisconsin | July 17, 2020 | Phase 3 |
| NCT04755764 | Recruiting | Drug: Labetalol Drug: Atenolol Drug: Nifedipine |
Systolic Hypertension Pregnancy Related |
Marshall University | March 3, 2021 | N/A |
| NCT05309460 | Not yet recruiting | Drug: Labetalol Oral Tablet Drug: NIFEdipine ER |
Postpartum Preeclampsia Hypertension in Pregnancy |
Nebraska Methodist Health System |
June 6, 2022 | Phase 4 |
| NCT06093893 | Not yet recruiting | Drug: Dexmedetomidine Drug: Nicardipine |
Hypotensive Anesthesia Orthognathic Surgery |
Boston Medical Center | March 2024 | Phase 4 |