| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Histamine H2 receptor
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:拉呋替丁是一种新开发的组胺H(2)受体拮抗剂,可抑制胃酸分泌。目前在日本(Stogar)、中国(乐美汀)和印度(Lafaxid)上市。它不仅抑制胃酸分泌,而且由于其诱导胃粘膜中胶原蛋白合成的特性而具有细胞保护特性。它具有新颖的作用机制,除了阻断 H2 受体外,还通过调节降钙素基因相关肽 (CGRP) 和香草酸受体来减轻炎症。它还被发现可以刺激粘蛋白生物合成并促进受损粘膜的恢复。拉呋替丁在小肠吸收,通过体循环到达胃细胞,然后直接快速地与胃细胞组胺H2受体结合,从而抑制cAMP的刺激和由此导致的产酸减少(抗分泌作用)。它导致内皮细胞内 Ca2+ 离子浓度持续增加,导致降钙素基因相关肽 (CGRP) 的释放,从而通过降低迷走神经张力来抑制酸。拉呋替丁还会增加血浆生长抑素水平,从而减少 G 细胞分泌胃泌素。胃泌素的减少会抑制壁细胞,导致胃酸分泌减少。
|
| 体内研究 (In Vivo) |
拉呋替丁(3-30 mg/kg;口服;每天两次;持续 6 天)可显着减轻结肠长度和髓过氧化物酶 (MPO) 活性的变化,并以剂量依赖性方式降低葡聚糖硫酸钠 (DSS) 诱导的结肠炎的严重程度方式[3]。
拉福替丁是一种组胺H(2)受体拮抗剂,具有与辣椒素敏感神经激活相关的胃抗分泌和胃保护活性。本研究探讨了拉福替丁对大鼠肠系膜阻力动脉中含辣椒素敏感降钙素基因相关肽(CGRP)的血管舒张神经(CGR能神经)神经传递的影响。用Krebs溶液灌注大鼠肠系膜血管床,用脱氧胆酸钠灌注30秒去除血管内皮。在通过连续灌注甲氧肟(α(1)肾上腺素受体激动剂)预收缩的制剂中,灌注拉氟替丁(0.1-10微M)浓度依赖性地增强了动脉周围神经刺激(PNS,1 Hz)诱导的血管舒张,而不影响外源性CGRP(10 pmol)注射诱导的血管扩张。法莫替丁(H(2)受体拮抗剂,1-100microM)的灌注对PNS诱导或CGRP诱导的血管舒张没有影响。通过大剂量注射辣椒素(香草醛-1受体激动剂,30pmol),拉福替丁的灌注浓度依赖性地增强了血管舒张作用。香草酸-1受体拮抗剂钌红(10微M)或辣椒素(5微M)的存在消除了辣椒素诱导的血管舒张,并显著降低了PNS诱导的血管扩张。钌红或辣椒素引起的PNS诱导的血管舒张减少不受拉福替丁灌注的影响。这些结果表明,拉福替丁通过调节位于CGR能神经中的突触前香草酸-1受体的功能来促进CGRP神经介导的血管舒张[2]。 |
| 细胞实验 |
拉福替丁是一种组胺H(2)受体拮抗剂,除了具有胃抗分泌活性外,还具有胃保护作用。拉福替丁的胃肠道保护作用是由辣椒素敏感神经元介导的,辣椒素通过打开瞬时受体电位通道家族(TRPV1)的成员来兴奋神经元。由于拉福替丁对细胞内钙离子浓度([Ca(2+)](i))的影响尚未阐明,我们研究了拉福替丁对大鼠嗜铬细胞瘤PC12和人内皮细胞中[Ca(2+)](i)的反应。在PC12细胞中存在细胞外CaCl(2)的情况下,药理学浓度大于1 mM的拉福替丁诱导[Ca(2+)](i)持续增加,而辣椒素对PC12细胞中的[Ca(2+)](ii)显示出双重作用,它激活TRPV1并抑制储存操作的Ca(2+)进入。辣椒素和SKF96365抑制了thapsigargin(一种储存操作Ca(2+)进入的激活剂)诱导的PC12细胞中[Ca(2+)](i)的增加,SKF96365-一种储存控制Ca(3+)进入的抑制剂,辣椒素抑制了拉福替丁的反应,但SKF96365.没有。在内皮细胞中,拉福替丁以SKF96365不敏感的方式诱导[Ca(2+)](i)的增加。这些结果表明,拉福替丁通过辣椒素敏感途径刺激钙进入,但不通过SKF96365敏感途径。还讨论了拉福替丁诱导的储存性Ca(2+)进入对胃肠功能的可能作用[4]。
|
| 动物实验 |
Male Wistar rats (180-200 g)
3 mg/kg, 10 mg/kg, 30 mg/kg Oral administration, twice daily, for 6 days The ligation of both the pylorus and the forestomach of SD rats under anesthesia caused hemorrhagic lesions in the esophageal mucosa at 6 h. Lesion formation was significantly inhibited by treatment with H(2)RAs, including the conventional H(2)RAs famotidine and cimetidine as well as lafutidine. The maximum suppressive abilities of these agents were similar to that of the proton pump inhibitor lansoprazole. Interestingly, unlike famotidine, lafutidine at low doses significantly suppressed esophagitis without inhibiting gastric acid secretion. Note that neither lafutidine nor famotidine inhibited hexosamine output in gastric juice samples obtained 3 h after ligation. Additionally, the protective effect of lafutidine, but not of famotidine, was partly attenuated by the denervation of capsaicin-sensitive afferent nerves with a large dose of capsaicin. [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
rat LD50 oral 1248 mg/kg BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX); BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS Oyo Yakuri. Pharmacometrics., 50(143), 1995
rat LD50 intravenous 84 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION Oyo Yakuri. Pharmacometrics., 50(143), 1995 mouse LD50 oral 1034 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION Oyo Yakuri. Pharmacometrics., 50(143), 1995 mouse LD50 intravenous 47900 ug/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION Oyo Yakuri. Pharmacometrics., 50(143), 1995 dog LD oral >400 mg/kg BEHAVIORAL: TREMOR; BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: NAUSEA OR VOMITING Oyo Yakuri. Pharmacometrics., 50(417), 1995 |
| 参考文献 |
|
| 其他信息 |
Lafutidine is an organic molecular entity.
Lafutidine has been investigated in Peptic Ulcer, Community-acquired Pneumonia, and Gastroesophageal Reflux Disease (GERD). Lafutidine, a histamine H2-receptor antagonist, exhibits gastric mucosal protective action mediated by capsaicin-sensitive afferent neurons, in addition to a potent antisecretory effect. In this study we examined the effect of lafutidine on dextran sulfate Na (DSS)-induced ulcerative colitis in rats, in relation to capsaicin-sensitive afferent neurons. Experimental colitis was induced in rats by daily treatment with 3% DSS in drinking water for 7 days. Lafutidine, capsaicin, and cimetidine were administered per os twice daily for 6 days. The ulceration area, colon length, and myeloperoxidase (MPO) activity were measured on day 7 after the onset of DSS treatment. DSS caused severe mucosal lesions in the colon, accompanied by an increase in MPO activity as well as a decrease in body weight gain and colon length. Daily administration of lafutidine dose-dependently reduced the severity of DSS-induced colitis and significantly mitigated changes in the colon length and MPO activity. The effects of lafutidine were mimicked by daily administration of capsaicin but not cimetidine and were totally abolished by chemical ablation of capsaicin-sensitive afferent neurons. In contrast, desensitization of afferent neurons significantly worsened the colonic inflammation induced by DSS. It was also found that both lafutidine and capsaicin increased the secretion of mucus in the colonic mucosa. These results suggest that lafutidine is effective against the ulcerative colitis induced by DSS through capsaicin-sensitive afferent neurons. This action might be attributable at least partly to the enhancement of colonic mucus secretion. [3] Gastroesophageal reflux disease is considered to be caused primarily by gastric juice refluxed into the esophagus. Here, we investigated the possible involvement of host defense mechanisms in the development of acute reflux esophagitis using lafutidine, a histamine H(2) receptor antagonist (H(2)RA) with proven gastric mucosal protective effects. Conclusion: The present results indicate that esophageal host-defense via capsaicin-sensitive afferent nerves may contribute to the therapeutic action of lafutidine. [1] |
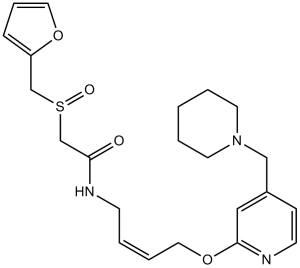
| 分子式 |
C22H29N3O4S
|
|---|---|
| 分子量 |
431.55
|
| 精确质量 |
431.187
|
| 元素分析 |
C, 61.23; H, 6.77; N, 9.74; O, 14.83; S, 7.43
|
| CAS号 |
118288-08-7
|
| 相关CAS号 |
(Z)-Lafutidine; 206449-93-6; Lafutidine-d10; 1795136-26-3; 118288-08-7; 169899-19-8
|
| PubChem CID |
5282136
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
704.2±60.0 °C at 760 mmHg
|
| 熔点 |
99 °C
|
| 闪点 |
379.7±32.9 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.599
|
| LogP |
1.1
|
| tPSA |
103.88
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
569
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C([H])([H])C(N([H])C([H])([H])/C(/[H])=C(/[H])\C([H])([H])OC1C([H])=C(C([H])=C([H])N=1)C([H])([H])N1C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O)(C([H])([H])C1=C([H])C([H])=C([H])O1)=O
|
| InChi Key |
KMZQAVXSMUKBPD-DJWKRKHSSA-N
|
| InChi Code |
InChI=1S/C22H29N3O4S/c26-21(18-30(27)17-20-7-6-14-28-20)23-9-2-5-13-29-22-15-19(8-10-24-22)16-25-11-3-1-4-12-25/h2,5-8,10,14-15H,1,3-4,9,11-13,16-18H2,(H,23,26)/b5-2-
|
| 化学名 |
2-(furan-2-ylmethylsulfinyl)-N-[(Z)-4-[4-(piperidin-1-ylmethyl)pyridin-2-yl]oxybut-2-enyl]acetamide
|
| 别名 |
FRG-8813; Lafutidine; FRG8813; 118288-08-7; 206449-93-6; rac Lafutidine; FRG-8813; (Z)-Lafutidine; Lafutidine [INN]; Lafutidine [JAN]; FRG 8813; trade name: Protecadin; Stogar
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (6.95 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3 mg/mL (6.95 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 30.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3 mg/mL (6.95 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3172 mL | 11.5861 mL | 23.1723 mL | |
| 5 mM | 0.4634 mL | 2.3172 mL | 4.6345 mL | |
| 10 mM | 0.2317 mL | 1.1586 mL | 2.3172 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Early effects of oral administrations of lafutidine with mint oil on intragastric pH
CTID: UMIN000001864
Phase: Status: Complete: follow-up complete
Date: 2009-05-01