| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Dopamine receptor
Dopamine D1 receptor (D1R) (EC50=120 nM for dopamine conversion-mediated activation) [4] Dopamine D2 receptor (D2R) (EC50=95 nM for dopamine conversion-mediated activation) [4] Mycobacterium tuberculosis (MIC=12.5 μg/mL) [2] |
|---|---|
| 体外研究 (In Vitro) |
左旋多巴在 25-200 μM 浓度下,在胎鼠中脑培养物中产生剂量依赖性的 3H-DA 摄取减少。左旋多巴会导致活细胞和酪氨酸羟化酶 (TH) 阳性神经元数量减少,并破坏整个神经炎网络。在缺乏多巴胺的情况下,左旋多巴通过过度抑制壳核-苍白球 (GPe) 投射的神经元以及随后对苍白球 (GPe) 的去抑制来诱发运动障碍。左旋多巴导致苍白球 (GPi) 中细胞色素氧化酶信使 RNA 表达减少。
MN9D多巴胺能神经元细胞经左旋多巴(Levodopa; L-DOPA)(10 μM-100 μM)处理后,通过细胞内多巴脱羧酶转化为多巴胺,50 μM时多巴胺释放增加3.2倍,MPP+损伤细胞的存活率提高45%(MTT法)[4] - 结核分枝杆菌(H37Rv菌株)经左旋多巴(Levodopa; L-DOPA)(1 μg/mL-64 μg/mL)处理后,展现抗菌活性,MIC=12.5 μg/mL,25 μg/mL时抑制细菌生长70%,减少55%的分枝杆菌生物膜形成[2] - 原代大鼠皮质神经元经左旋多巴(Levodopa; L-DOPA)(20 μM-100 μM)处理后,60 μM时减少58%的谷氨酸诱导兴奋性毒性(LDH法),降低52%的活性氧(ROS)产生,谷胱甘肽(GSH)水平升高1.8倍[3] - 人红细胞裂解液与左旋多巴(Levodopa; L-DOPA)(50 μM-500 μM)孵育后,200 μM时经内源性多巴脱羧酶代谢为多巴胺,转化率达38%[5] |
| 体内研究 (In Vivo) |
左旋多巴会引起由神经毒素 MPTP 诱发的帕金森病猴子出现多种异常运动。左旋多巴给药导致 6-OHDA 损伤大鼠 CdPu 中多巴胺 D3 受体表达的异位诱导。左旋多巴 (50 mg/kg) 通过激活完整大鼠的多巴胺 D1/D2 受体,增加整个基底神经节的 anandamide 浓度。左旋多巴在病变大鼠中产生越来越严重的口舌不自主运动,这种运动被大麻素激动剂 R(+)-WIN55,212-2 (1 mg/kg) 减弱。左旋多巴给药可逆转严重病变大鼠中 D2 多巴胺受体的上调,这提供了左旋多巴在基底神经节达到生物活性浓度的证据。
6-羟基多巴胺(6-OHDA)诱导大鼠帕金森病(PD)模型:腹腔注射左旋多巴(Levodopa; L-DOPA)(10 mg/kg、20 mg/kg、40 mg/kg),每日一次,连续21天。40 mg/kg剂量改善旋转行为72%(阿扑吗啡诱导旋转实验),纹状体多巴胺水平升高2.5倍(HPLC检测)[1] - PD患者临床试验:口服左旋多巴(Levodopa; L-DOPA)(100 mg/次,每日3次)联合卡比多巴(25 mg/次,每日3次),连续12周,与基线相比,统一帕金森病评定量表(UPDRS)运动评分改善50%。震颤和僵硬显著缓解,30分钟内起效[5] - 1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)诱导小鼠PD模型:口服灌胃左旋多巴(Levodopa; L-DOPA)(25 mg/kg)+苄丝肼(10 mg/kg),每日两次,连续14天,逆转运动缺陷(爬杆实验潜伏期缩短60%),保护黑质多巴胺能神经元(神经元丢失减少48%)[4] - 小鼠结核分枝杆菌感染模型:腹腔注射左旋多巴(Levodopa; L-DOPA)(50 mg/kg、100 mg/kg),每周3次,连续4周。100 mg/kg剂量减少肺组织细菌载量62%,脾组织细菌载量58%[2] |
| 酶活实验 |
多巴脱羧酶活性实验:制备大鼠脑匀浆(多巴脱羧酶来源),与左旋多巴(Levodopa; L-DOPA)(50 μM-500 μM)在含磷酸吡哆醛的缓冲液中37°C孵育60分钟。高效液相色谱(HPLC)电化学检测多巴胺生成量,计算转化率[5]
- 分枝杆菌生长抑制实验:在Middlebrook 7H9培养基中制备左旋多巴(Levodopa; L-DOPA)系列稀释液(1 μg/mL-64 μg/mL),接种结核分枝杆菌(10⁶ CFU/mL),37°C孵育7天。600 nm吸光度检测细菌生长,以最低抑制浓度为MIC[2] |
| 细胞实验 |
左旋多巴是一种用于帕金森病(PD)患者的多巴胺(DA)前体,在25-200 x 10(-6)M的浓度下,会导致胎鼠中脑培养物中3H-DA摄取的剂量依赖性减少。此外,在培养基中醌水平升高的同时,观察到活细胞和酪氨酸羟化酶(TH)阳性神经元数量的减少,以及整个神经网络的破坏。抗坏血酸(AA)消除了醌的过度生产,部分阻止了这些影响。尽管左旋多巴在体内的神经毒性尚未得到证实,但AA可能会降低PD患者内源性或移植DA神经元的脆弱性[1]。
多巴胺能神经元保护实验:将MN9D细胞接种于96孔板,孵育24小时后,用左旋多巴(Levodopa; L-DOPA)(10 μM-100 μM)预处理1小时,再用MPP+(500 μM)处理24小时。MTT法评估细胞活力;ELISA法检测上清液多巴胺浓度[4] - 神经元兴奋性毒性实验:原代大鼠皮质神经元接种于24孔板培养7天,用左旋多巴(Levodopa; L-DOPA)(20 μM-100 μM)预处理2小时,再用谷氨酸(100 μM)刺激24小时。LDH释放法评估细胞损伤;荧光探针检测ROS和GSH水平[3] |
| 动物实验 |
7-week-old C57BL/6J mice
20 mg/kg Orally Animal Surgery and Treatments. Wistar male rats (180–200 g, Iffa Credo) were anesthetized with pentobarbital (50 mg/kg, i.p.) and infused over 8 min with 6-OHDA (8 μg in 4 μl of 0.05% ascorbic acid in saline) at coordinates A = −3.8 mm, L = 1.5 mm, H = −8.5 mm. Three weeks later, they received twice a day, and for various periods of time, i.p. injections of vehicle, levodopa (in all experiments as l-DOPA methyl ester, 50 mg/kg, in combination with benserazide, a peripheral dopa decarboxylase inhibitor, 12.5 mg/kg) or levodopa plus SCH 23390 (0.5 mg/kg) or plus SKF 38393 (10 mg/kg), bromocriptine (10 mg/kg), quinpirole (0.1 mg/kg).[3] The majority of Parkinson's disease patients undergoing levodopa therapy develop disabling motor complications (dyskinesias) within 10 years of treatment. Stimulation of cannabinoid receptors, the pharmacological target of Delta 9-tetrahydrocannabinol, is emerging as a promising therapy to alleviate levodopa-associated dyskinesias. However, the mechanisms underlying this beneficial action remain elusive, as do the effects exerted by levodopa therapy on the endocannabinoid system. Although levodopa is known to cause changes in CB1 receptor expression in animal models of Parkinson's disease, we have no information on whether this drug alters the brain concentrations of the endocannabinoids anandamide and 2-arachidonylglycerol. To address this question, we used an isotope dilution assay to measure endocannabinoid levels in the caudate-putamen, globus pallidus and substantia nigra of intact and unilaterally 6-OHDA-lesioned rats undergoing acute or chronic treatment with levodopa (50 mg/kg). In intact animals, systemic administration of levodopa increased anandamide concentrations throughout the basal ganglia via activation of dopamine D1/D2 receptors. In 6-OHDA-lesioned rats, anandamide levels were significantly reduced in the caudate-putamen ipsilateral to the lesion; however, neither acute nor chronic levodopa treatment affected endocannabinoid levels in these animals. In lesioned rats, chronic levodopa produced increasingly severe oro-lingual involuntary movements which were attenuated by the cannabinoid agonist R(+)-WIN55,212-2 (1 mg/kg). This effect was reversed by the CB1 receptor antagonist rimonabant (SR141716A). These results indicate that a deficiency in endocannabinoid transmission may contribute to levodopa-induced dyskinesias and that these complications may be alleviated by activation of CB1 receptors.[4] Orally administered levodopa remains the most effective symptomatic treatment for Parkinson's disease (PD). The introduction of levodopa therapy is often delayed, however, because of the fear that it might be toxic for the remaining dopaminergic neurons and, thus, accelerate the deterioration of patients. However, in vivo evidence of levodopa toxicity is scarce. We have evaluated the effects of a 6-month oral levodopa treatment on several dopaminergic markers, in rats with moderate or severe 6-hydroxydopamine-induced lesions of mesencephalic dopamine neurons and sham-lesioned animals. Counts of tyrosine hydroxylase (TH)-immunoreactive neurons in the substantia nigra and ventral tegmental area showed no significant difference between levodopa-treated and vehicle-treated rats. In addition, for rats of the sham-lesioned and severely lesioned groups, immunoradiolabeling for TH, the dopamine transporter (DAT), and the vesicular monoamine transporter (VMAT2) at the striatal level was not significantly different between rats treated with levodopa or vehicle. It was unexpected that quantification of immunoautoradiograms showed a partial recovery of all three dopaminergic markers (TH, DAT, and VMAT2) in the denervated territories of the striatum of moderately lesioned rats receiving levodopa. Furthermore, the density of TH-positive fibers observed in moderately lesioned rats was higher in those treated chronically with levodopa than in those receiving vehicle. Last, that chronic levodopa administration reversed the up-regulation of D2 dopamine receptors seen in severely lesioned rats provided evidence that levodopa reached a biologically active concentration at the basal ganglia. Our results demonstrate that a pharmacologically effective 6-month oral levodopa treatment is not toxic for remaining dopamine neurons in a rat model of PD but instead promotes the recovery of striatal innervation in rats with partial lesions.[5] 6-OHDA-induced rat PD model: Male Sprague-Dawley rats (250-300 g) were anesthetized and injected with 6-OHDA into the right medial forebrain bundle. After 2 weeks of recovery, Levodopa (L-DOPA) was dissolved in physiological saline and administered via intraperitoneal injection (10 mg/kg, 20 mg/kg, 40 mg/kg) daily for 21 days. Evaluate rotational behavior 30 minutes post-administration; euthanize rats to measure striatal dopamine levels [1] - MPTP-induced mouse PD model: Male C57BL/6 mice (20-25 g) were intraperitoneally injected with MPTP (20 mg/kg) daily for 5 days to induce PD. From day 6, Levodopa (L-DOPA) (25 mg/kg) plus benserazide (10 mg/kg) was administered via oral gavage twice daily for 14 days. Perform pole test and rotarod test to assess motor function; immunostain nigral tissues for tyrosine hydroxylase (TH) to count dopamineergic neurons [4] - Mycobacterium tuberculosis mouse model: Female BALB/c mice (18-22 g) were intravenously infected with Mycobacterium tuberculosis (10⁵ CFU/mouse). Seven days post-infection, Levodopa (L-DOPA) was dissolved in 0.5% carboxymethylcellulose sodium and administered via intraperitoneal injection (50 mg/kg, 100 mg/kg) three times weekly for 4 weeks. Euthanize mice to quantify bacterial load in lungs and spleen [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Orally inhaled levodopa reaches a peak concentration in 0.5 hours with a bioavailability than is 70% that of the immediate release levodopa tablets with a peripheral dopa decarboxylase inhibitor like carbidopa or benserazide. After 48 hours, 0.17% of an orally administered dose is recovered in stool, 0.28% is exhaled, and 78.4% is recovered in urine 168L for orally inhaled levodopa. Intravenously administered levodopa is cleared at a rate of 14.2mL/min/kg in elderly patients and 23.4mL/min/kg in younger patients. When given carbidopa, the clearance of levodopa was 5.8mL/min/kg in elderyly patients and 9.3mL/min/kg in younger patients. ...DRUG...MAY APPEAR IN MILK. AFTER IP INJECTION INTO MICE, BIOTRANSFORMATION OF 60% OF RADIOACTIVELY LABELLED DL-DOPA TAKES PLACE WITHIN 10 MIN, & PEAK DOPAMINE LEVELS ARE REACHED 20 MIN AFTER ADMIN. ...APPROX 0.1% OF DOSE WAS PRESENT IN THE BRAIN AS (14)C-L-DOPA OR (14)C-DOPAMINE. /DL-DOPA/ MORE THAN 95% OF LEVODOPA IS DECARBOXYLATED IN PERIPHERY BY WIDELY DISTRIBUTED EXTRACEREBRAL AROMATIC L-AMINO ACID DECARBOXYLASE. ...LITTLE UNCHANGED DRUG REACHES CEREBRAL CIRCULATION & PROBABLY LESS THAN 1% PENETRATES INTO CNS. MOST IS CONVERTED TO DOPAMINE... DOPAMINE METABOLITES ARE RAPIDLY EXCRETED IN URINE, ABOUT 80% OF RADIOACTIVELY LABELED DOSE BEING RECOVERED WITHIN 24 HR. ... THESE METABOLITES /3,4-DIHYDROXYPHENYLACETIC ACID & 3-METHOXY-4-HYDROXYPHENYLACETIC ACID/, AS WELL AS SMALL AMT OF LEVODOPA & DOPAMINE, ALSO APPEAR IN CEREBROSPINAL FLUID. For more Absorption, Distribution and Excretion (Complete) data for LEVODOPA (11 total), please visit the HSDB record page. Metabolism / Metabolites Levodopa is either converted to dopamine by aromatic-L-amino-acid decarboxylase or O-methylated to 3-O-methyldopa by catechol-O-methyltransferase. 3-O-methyldopa cannot be metabolized to dopamine. Once levodopa is converted to dopamine, it is converted to sulfated or glucuronidated metabolites, epinephrine E, or homovanillic acid through various metabolic processes. The primary metabolites are 3,4-dihydroxyphenylacetic acid (13-47%) and homovanillic acid (23-39%). MOST IS CONVERTED TO DOPAMINE... BIOTRANSFORMATION OF DOPAMINE PROCEEDS RAPIDLY...EXCRETION PRODUCTS, 3,4-DIHYDROXYPHENYLACETIC ACID...& 3-METHOXY-4-HYDROXYPHENYLACETIC ACID... SOME BIOCHEMICAL EVIDENCE INDICATES THAT ACCELERATION OF LEVODOPA METABOLISM OCCURS DURING PROLONGED THERAPY, POSSIBLY DUE TO ENZYME INDUCTION. MORE THAN 95%...IS DECARBOXYLATED...BY...AROMATIC L-AMINO ACID DECARBOXYLASE. ... A SMALL AMT /OF L-DOPA/ IS METHYLATED TO 3-O-METHYL-DOPA... MOST IS CONVERTED TO DOPAMINE, SMALL AMT OF WHICH IN TURN ARE METABOLIZED TO NOREPINEPHRINE & EPINEPHRINE. ...IS ESTIMATED THAT ABOUT THREE FOURTHS OF DIETARY METHIONINE IS UTILIZED FOR METABOLISM OF LARGE THERAPEUTIC DOSES OF LEVODOPA. LEVODOPA (L-DOPA) IS FORMED IN MAMMALS FROM L-TYROSINE AS INTERMEDIARY METABOLITE IN ENZYMATIC SYNTHESIS OF CATECHOLAMINES. 95% of an administered oral dose of levodopa is pre-systemically decarboxylated to dopamine by the L-aromatic amino acid decarboxylase (AAAD) enzyme in the stomach, lumen of the intestine, kidney, and liver. Levodopa also may be methoxylated by the hepatic catechol-O-methyltransferase (COMT) enzyme system to 3-O-methyldopa (3-OMD), which cannot be converted to central dopamine. Half Life: 50 to 90 minutes Biological Half-Life 2.3 hours for orally inhaled levodopa. Oral levodopa has a half life of 50 minutes but when combined with a peripheral dopa decarboxylase inhibitor, the half life is increased to 1.5 hours. THE HALF-LIFE IN PLASMA IS SHORT (1-3 HR). Absorption: Oral bioavailability is 30-40% in humans (alone); increases to 70-80% when co-administered with carbidopa (peripheral dopa decarboxylase inhibitor). Peak plasma concentration (Cmax) is reached at 1-2 hours post-oral administration (100 mg dose: Cmax=1.2 μg/mL) [5] - Distribution: Volume of distribution (Vd) is 0.8-1.2 L/kg in humans; penetrates the blood-brain barrier (BBB) via large neutral amino acid transporter (LAT1), with brain/plasma concentration ratio=0.1-0.2 [5] - Metabolism: Rapidly metabolized by dopa decarboxylase (peripheral and central) to dopamine; also metabolized via catechol-O-methyltransferase (COMT) to 3-O-methyldopa (inactive) [5] - Excretion: 80% of metabolites are excreted in urine, 10% in feces. Elimination half-life (t1/2) is 1-2 hours in humans (alone), prolonged to 2-3 hours with carbidopa [5] - Plasma protein binding: Levodopa (L-DOPA) has a plasma protein binding rate of 10-15% in human plasma [5] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited data indicate that levodopa is poorly excreted into breastmilk and that the sustained-release product may result in a smaller amount of drug transferred to the breastfed infant than with the immediate-release product. Several studies indicate that levodopa can decrease serum prolactin during lactation. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. The effect of long-term use of levodopa on breastfeeding has not been adequately evaluated, although some mothers were able to successfully breastfeed her infant without apparent harm while using relatively low doses of levodopa and carbidopa for Parkinson's disease. ◉ Effects in Breastfed Infants One mother with Parkinson's disease took sustained-release levodopa 200 mg and carbidopa 50 mg 4 times daily. She successfully breastfed her infant whose development was normal at 2 years of age. A 37-year-old Israeli woman with Parkinson's disease became pregnant while taking a continuous infusion of levodopa 20 mg/mL and carbidopa 5 mg/mL gel. She breastfed her infant for 3 months while receiving the drug, although the extent of breastfeeding and the dosage of the gel is not clear from the paper. At 10 months of age, the infant's psychomotor development was deemed to be normal. ◉ Effects on Lactation and Breastmilk Levodopa decreases serum prolactin in normal women and those with hyperprolactinemia and can suppress inappropriate lactation in galactorrhea, although not consistently. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. One mother with Parkinson's disease took sustained-release levodopa 200 mg and carbidopa 50 mg 4 times daily. She successfully breastfed her infant. On postpartum day 3, 5 women were given a single oral dose of 500 mg of levodopa or bromocriptine 5 mg followed by a single oral dose of metoclopramide 10 mg 3 hours later. Bromocriptine suppressed basal serum prolactin to a greater extent than levodopa. Over the next 3 hours, serum prolactin increased after metoclopramide in the patients who received levodopa, but not in those who received bromocriptine. Six women who were 2 to 4 days postpartum, but were not nursing, were given 500 mg of levodopa orally on one day and 100 mg of levodopa plus 35 mg of carbidopa orally on the next day. Both regimens suppressed basal serum prolactin levels. However, levodopa alone caused an 78% decrease in prolactin while the lower dose combination produced only a 51% decrease. The maximal effect occurred about 2 hours after the dose with both regimens. Seven women in the first week postpartum who were breastfeeding about 7 times daily were given levodopa 500 mg orally and their serum prolactin responses was studied. The following day, they started carbidopa 50 mg orally every 6 hours for 2 days. On the third day, they received a single dose of carbidopa 50 mg plus levodopa 125 mg orally. Decreases in basal serum prolactin occurred by 30 minutes after the levodopa and after 45 minutes with the combination. Decreases were maximum at 120 minutes after the dose and were 62% with levodopa alone and 48% with the combination, although the difference between the 2 regimens was not statistically significant. A 37-year-old Israeli woman with Parkinson's disease became pregnant while taking a continuous infusion of levodopa 20 mg/mL and carbidopa 5 mg/mL gel. She breastfed her infant for 3 months while receiving the drug, although the extent of breastfeeding and the dosage of the gel is not clear from the paper. Protein Binding Levodopa binding to plasma proteins is negligible. Acute toxicity: LD50 is 1890 mg/kg (oral) in rats, 1780 mg/kg (oral) in mice [1] - Chronic toxicity: Rats administered Levodopa (L-DOPA) (200 mg/kg/day, oral) for 6 months showed increased locomotor activity and mild intestinal hyperplasia, no significant liver/kidney toxicity [1] - Clinical side effects: Motor complications (dyskinesia, wearing-off phenomenon) in 50-60% of patients after long-term use (>5 years); gastrointestinal symptoms (nausea, vomiting, diarrhea) in 30-40% (reduced with carbidopa); psychiatric symptoms (hallucinations, delusions) in 10-15% [5] - Drug-drug interaction: Co-administration with carbidopa/benserazide (peripheral dopa decarboxylase inhibitors) reduces peripheral metabolism and side effects; COMT inhibitors (e.g., entacapone) prolong half-life; MAO inhibitors (e.g., phenelzine) increase risk of hypertensive crisis [5] |
| 参考文献 | |
| 其他信息 |
Levodopa can cause developmental toxicity according to state or federal government labeling requirements.
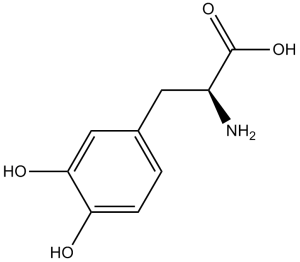
L-dopa is an optically active form of dopa having L-configuration. Used to treat the stiffness, tremors, spasms, and poor muscle control of Parkinson's disease It has a role as a prodrug, a hapten, a neurotoxin, an antiparkinson drug, a dopaminergic agent, an antidyskinesia agent, an allelochemical, a plant growth retardant, a human metabolite, a mouse metabolite and a plant metabolite. It is a dopa, a L-tyrosine derivative and a non-proteinogenic L-alpha-amino acid. It is a conjugate acid of a L-dopa(1-). It is an enantiomer of a D-dopa. It is a tautomer of a L-dopa zwitterion. Levodopa is a prodrug of dopamine that is administered to patients with Parkinson's due to its ability to cross the blood-brain barrier. Levodopa can be metabolised to dopamine on either side of the blood-brain barrier and so it is generally administered with a dopa decarboxylase inhibitor like carbidopa to prevent metabolism until after it has crossed the blood-brain barrier. Once past the blood-brain barrier, levodopa is metabolized to dopamine and supplements the low endogenous levels of dopamine to treat symptoms of Parkinson's. The first developed drug product that was approved by the FDA was a levodopa and carbidopa combined product called Sinemet that was approved on May 2, 1975. 3,4-Dihydroxy-L-phenylalanine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Levodopa is an Aromatic Amino Acid. Levodopa has been reported in Mucuna macrocarpa, Amanita muscaria, and other organisms with data available. Levodopa is an amino acid precursor of dopamine with antiparkinsonian properties. Levodopa is a prodrug that is converted to dopamine by DOPA decarboxylase and can cross the blood-brain barrier. When in the brain, levodopa is decarboxylated to dopamine and stimulates the dopaminergic receptors, thereby compensating for the depleted supply of endogenous dopamine seen in Parkinson's disease. To assure that adequate concentrations of levodopa reach the central nervous system, it is administered with carbidopa, a decarboxylase inhibitor that does not cross the blood-brain barrier, thereby diminishing the decarboxylation and inactivation of levodopa in peripheral tissues and increasing the delivery of dopamine to the CNS. L-Dopa is used for the treatment of Parkinsonian disorders and Dopa-Responsive Dystonia and is usually given with agents that inhibit its conversion to dopamine outside of the central nervous system. Peripheral tissue conversion may be the mechanism of the adverse effects of levodopa. It is standard clinical practice to co-administer a peripheral DOPA decarboxylase inhibitor - carbidopa or benserazide - and often a catechol-O-methyl transferase (COMT) inhibitor, to prevent synthesis of dopamine in peripheral tissue. The naturally occurring form of dihydroxyphenylalanine and the immediate precursor of dopamine. Unlike dopamine itself, it can be taken orally and crosses the blood-brain barrier. It is rapidly taken up by dopaminergic neurons and converted to dopamine. It is used for the treatment of parkinsonian disorders and is usually given with agents that inhibit its conversion to dopamine outside of the central nervous system. [PubChem] L-Dopa is the naturally occurring form of dihydroxyphenylalanine and the immediate precursor of dopamine. Unlike dopamine itself, L-Dopa can be taken orally and crosses the blood-brain barrier. It is rapidly taken up by dopaminergic neurons and converted to dopamine. In particular, it is metabolized to dopamine by aromatic L-amino acid decarboxylase. Pyridoxal phosphate (vitamin B6) is a required cofactor for this decarboxylation, and may be administered along with levodopa, usually as pyridoxine. The naturally occurring form of DIHYDROXYPHENYLALANINE and the immediate precursor of DOPAMINE. Unlike dopamine itself, it can be taken orally and crosses the blood-brain barrier. It is rapidly taken up by dopaminergic neurons and converted to DOPAMINE. It is used for the treatment of PARKINSONIAN DISORDERS and is usually given with agents that inhibit its conversion to dopamine outside of the central nervous system. See also: Melevodopa (is active moiety of); Carbidopa; Levodopa (component of); Carbidopa; entacapone; levodopa (component of) ... View More ... Drug Indication Levodopa on its own is formulated as an oral inhalation powder indicated for intermittent treatment of off episodes in Parkinson's patients who are already being treated with carbidopa and levodopa. Levodopa is most commonly formulated as an oral tablet with a peripheral dopa decarboxylase inhibitor indicated for treatment of Parkinson's disease, post-encephalitic parkinsonism, and symptomatic parkinsonism following carbon monoxide intoxication or manganese intoxication. FDA Label Inbrija is indicated for the intermittent treatment of episodic motor fluctuations (OFF episodes) in adult patients with Parkinson's disease (PD) treated with a levodopa/dopa-decarboxylase inhibitor. Treatment of Parkinson's disease Mechanism of Action Levodopa by various routes crosses the blood brain barrier, is decarboxylated to form dopamine. This supplemental dopamine performs the role that endogenous dopamine cannot due to a decrease of natural concentrations and stimulates dopaminergic receptors. MOST WIDELY ACCEPTED THEORY IS THAT LEVODOPA INCR LEVEL OF DOPAMINE & THUS ACTIVATION OF DOPAMINE RECEPTORS IN EXTRA-PYRAMIDAL CENTERS IN THE BRAIN (PRIMARILY IN CAUDATE NUCLEUS & SUBSTANTIA NIGRA). The present data indicate that the major effects observed after administration of exogenous levodopa are not due to a direct action of levodopa on dopamine receptors, or to extrastriatal release of dopamine, but to conversion of levodopa to dopamine by serotonergic terminals and probably some intrastriatal cells. EFFECTS OF LEVODOPA ON HUMAN & MURINE MELANOMA CELLS EXAMINED. WHEN EXPONENTIALLY GROWING CELLS WERE EXPOSED TO L-DOPA, CHARACTERISTIC INHIBITION OF THYMIDINE INCORPORATION OBSERVED. IN RATS, DOPAMINERGIC AGONISTS ALL CAUSED DECR IN SERUM PROLACTIN LEVELS. Levodopa (L-DOPA) is the precursor of dopamine, a central nervous system (CNS) neurotransmitter, with neuroprotective and antibacterial activities [1,2,3,4,5] Its core mechanisms include conversion to dopamine (via dopa decarboxylase) to supplement depleted dopamine in PD patients, protection of dopamineergic neurons from excitotoxicity/oxidative stress, and inhibition of Mycobacterium tuberculosis growth [1,4,5] Indications include Parkinson's disease (primary treatment for motor symptoms: tremor, rigidity, bradykinesia) and dopa-responsive dystonia [5] Peripheral metabolism limits BBB penetration and causes side effects, so it is almost always co-administered with peripheral dopa decarboxylase inhibitors (carbidopa/benserazide) [5] Short half-life requires multiple daily dosing (3-4 times/day) or controlled-release formulations to maintain stable plasma concentrations [5] It exhibits antibacterial activity against Mycobacterium tuberculosis, suggesting potential as an adjuvant in tuberculosis treatment (clinical validation needed) [2] Long-term use is associated with motor complications, requiring dose adjustment or combination with other PD drugs (e.g., dopamine agonists) [5] |
| 分子式 |
C9H11NO4
|
|
|---|---|---|
| 分子量 |
197.19
|
|
| 精确质量 |
197.068
|
|
| 元素分析 |
C, 54.82; H, 5.62; N, 7.10; O, 32.46
|
|
| CAS号 |
59-92-7
|
|
| 相关CAS号 |
L-DOPA-2,5,6-d3; 53587-29-4; L-DOPA-d6; 713140-75-1; L-DOPA sodium; 63302-01-2; L-DOPA-13C6; 201417-12-1; L-DOPA-13C; 586971-29-1
|
|
| PubChem CID |
6047
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
448.4±45.0 °C at 760 mmHg
|
|
| 熔点 |
276-278 °C(lit.)
|
|
| 闪点 |
225.0±28.7 °C
|
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
|
| 折射率 |
1.655
|
|
| LogP |
-0.22
|
|
| tPSA |
103.78
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
209
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
O([H])C1=C(C([H])=C([H])C(=C1[H])C([H])([H])[C@@]([H])(C(=O)O[H])N([H])[H])O[H]
|
|
| InChi Key |
WTDRDQBEARUVNC-LURJTMIESA-N
|
|
| InChi Code |
InChI=1S/C9H11NO4/c10-6(9(13)14)3-5-1-2-7(11)8(12)4-5/h1-2,4,6,11-12H,3,10H2,(H,13,14)/t6-/m0/s1
|
|
| 化学名 |
(2S)-2-amino-3-(3,4-dihydroxyphenyl)propanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 (2). 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 3.33 mg/mL (16.89 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0713 mL | 25.3563 mL | 50.7125 mL | |
| 5 mM | 1.0143 mL | 5.0713 mL | 10.1425 mL | |
| 10 mM | 0.5071 mL | 2.5356 mL | 5.0713 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04990284 | Active Recruiting |
Drug: Opicapone Drug: L-DOPA/DDCI |
Parkinson Disease | Bial - Portela C S.A. | November 29, 2021 | Phase 4 |
| NCT02480803 | Active Recruiting |
Device: deep brain stimulation Drug: Continuous intrajejunal infusion of levodopa-carbidopa |
Parkinson's Disease | Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) |
December 19, 2014 | Phase 4 |
| NCT03243552 | Active Recruiting |
Drug: L-DOPA versus Placebo Behavioral: Social Skills Training |
ASD | University of California, Los Angeles |
June 1, 2017 | Phase 2 |
| NCT04469959 | Recruiting | Drug: L-Dopa Drug: Placebo |
Levodopa Gait Impairment |
Vanderbilt University Medical Center |
February 15, 2021 | Phase 2 |
| NCT06075771 | Recruiting | Drug: Carbidopa Levodopa Drug: Placebo |
Anhedonia Depression |
Emory University | November 21, 2023 | Phase 4 |
|
|
|