| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:与有氧条件下相比,氮芥在氮气条件下对大鼠肝细胞的毒性要小得多,并且引起的脂质过氧化要少得多。氮芥显着抑制细胞生长,并导致与兔气管原代培养物中细胞骨架蛋白重排相关的细胞脱离。二氯乙胺导致兔气管原代培养物中早期脂质过氧化和细胞膜损伤,这与抗氧化酶活性显着增加相关,而抗氧化酶活性显着增加,而抗氧化酶活性显着增加,而抗氧化酶活性显着增加,而谷胱甘肽含量增加
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
MeChlorethamine (1.5 mg/kg iv) 可将兔子的平均白细胞计数从 6,320 mm3 降低至 1,890 mm3,但对白细胞分类或红细胞和血小板计数没有任何变化。氮芥(0.005 mg-0.5 mg,id)会引起小鼠剂量依赖性皮肤溃疡,在氮芥后立即给予等渗硫代硫酸钠(0.167 M)或高渗(0.34 M)(0.05 mL)可显着减少平均 HN2 溃疡面积和溃疡的总时间。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following IV injection, the drug undergoes rapid chemical transformation and unchanged mechlorethamine is undetectable in the blood within a few minutes. Less than 0.01% of an IV dose is excreted unchanged in the urine. Mice given 35 mg/kg body wt mechlorethamine hydrochloride iv and examined by autoradiography had significant levels of compound in brain, spinal cord, lung and submaxillary glands. Mechlorethamine is incompletely absorbed following intracavitary administration, probably because of rapid deactivation by body fluids. Metabolism / Metabolites Following its in vivo admin, mechlorethamine or its hydrochloride is probably converted into ethyleneimmonium ion which reacts with guanine residues in /either the same or/ adjacent strands of DNA as well as with SH groups. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Most sources consider that mothers receiving antineoplastic therapy should not breastfeed, especially with alkylating agents such as mechlorethamine. Because of the potential for serious adverse reactions in the breastfed infant, breastfeeding from mechlorethamine mothers should not breastfed during therapy with mechlorethamine therapy, including topical application. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions Morphine and pethidine hydrochloride significantly potentiated mechlorethamine hydrochloride toxicity. The potentiation seems dose related. Morphine converted sublethal doses of mechlorethamine to maximal LD's. In HT29 human colon carcinoma cells, amphotericin b (approx 120 ug/ml) increased uptake of mechlorethamine hydrochloride due to increases in vmax without change in km. Mechlorethamine may raise the concentration of blood uric acid; dosage adjustment of antigout agents may be necessary to control hyperuricemia and gout; allopurinol may be preferred to prevent or reverse mechlorethamine-induced hyperuricemia because of risk of uric acid nephropathy with uricosuric antigout agents. Leukopenic and/or thrombocytopenic effects of mechlorethamine may be increased with concurrent or recent therapy /with blood dyscrasia-causing medications/ if these medications cause the same effects; dosage adjustment of mechlorethamine, if necessary, should be based on blood counts. For more Interactions (Complete) data for MECHLORETHAMINE HYDROCHLORIDE (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 1.1 mg/kg LD50 Rat sc 1.9 mg/kg |
||
| 参考文献 | |||
| 其他信息 |
Nitrogen Mustard Hydrochloride (Mechlorethamine Hydrochloride) can cause cancer according to an independent committee of scientific and health experts. It can cause developmental toxicity according to state or federal government labeling requirements.
Nitrogen mustard hydrochloride appears as white to off-white crystals or powder with a fishy odor. Initial pH (2% aqueous solution) 3.0-4.0. (NTP, 1992) Mechlorethamine hydrochloride is the hydrochloride salt of mechlorethamine. It has a role as an antineoplastic agent. It contains a mechlorethamine. Mechlorethamine Hydrochloride is the hydrochloride salt of mechlorethamine, a nitrogen mustard and an analogue of sulfur mustard, with antineoplastic and immunosuppressive activities. Mechlorethamine is metabolized to an unstable, highly reactive ethyleniminium intermediate that alkylates DNA, particularly the 7 nitrogen of guanine residues, resulting in DNA base pair mismatching, DNA interstrand crosslinking, the inhibition of DNA repair and synthesis, cell-cycle arrest, and apoptosis. This agent also exhibits lympholytic properties. A biologic alkylating agent that exerts its cytotoxic effects by forming DNA ADDUCTS and DNA interstrand crosslinks, thereby inhibiting rapidly proliferating cells. The hydrochloride is an antineoplastic agent used to treat HODGKIN DISEASE and LYMPHOMA. Mechanism of Action Mechlorethamine, as an alkylating agent, interferes with DNA replication and transcription of RNA and ultimately results in the disruption of nucleic acid function. Mechlorethamine also possesses weak immunosuppressive activity. Mechlorethamine, as an alkylating agent, interferes with DNA replication and transcription of RNA, and ultimately results in the disruption of nucleic acid function. Therapeutic Uses Alkylating Agents; Antineoplastic Agents, Alkylating; Antineoplastic VET: Antineoplastic MEDICATION (VET): ... MECHLORETHAMINE HYDROCHLORIDE IS REPORTED TO BE USED FOR TREATMENT OF LYMPHOSARCOMA & OF MAST CELL SARCOMA IN DOGS & OF FOWL LEUKOSIS ... /MECHLORETHAMINE HYDROCHLORIDE/ For more Therapeutic Uses (Complete) data for MECHLORETHAMINE HYDROCHLORIDE (11 total), please visit the HSDB record page. Drug Warnings Adverse CNS effects which have occurred following IV administration of mechlorethamine include weakness, headache, drowsiness, vertigo, lightheadedness, convulsions, progressive muscle paralysis, paresthesia, cerebral degeneration, coma, and death. Serious neurotoxicity appears to be a problem only when high doses or intra-arterial and regional perfusion administration techniques are used. Immediate and delayed neurotoxicity, sometimes severe, has been reported in patients receiving higher than recommended doses of the drug in preparation for bone marrow transplantation; neurotoxicity appeared to increase with age and dose administered and occurred more frequently in patients who also received procarbazine or cyclophosphamide. Adverse dermatologic effects of systemic mechlorethamine therapy occasionally include a maculopapular skin eruption which is apparently idiosyncratic. The maculopapular skin eruption does not necessarily recur with subsequent doses and is not a contraindication to future use of the drug. Erythema multiforme also has been reported. Hypersensitivity reactions, including anaphylaxis, have been reported in patients receiving IV mechlorethamine. ... Herpes zoster, which occurs commonly in patients with lymphoma, may be precipitated by treatment with mechlorethamine. Major and dose-limiting adverse effects of mechlorethamine are nausea and vomiting, which occur in up to 90% of patients who receive the drug and are presumably due to CNS stimulation.Vomiting, which may be severe enough to precipitate vascular accidents in patients with hemorrhagic tendencies, occurs within 0.5-8 hours (usually 1-3 hours) after administration of mechlorethamine. Emesis generally subsides within 8 hours, but nausea may persist 24 hours or longer. ... Other GI effects of mechlorethamine include anorexia, diarrhea, severe hematemesis and dehydration secondary to vomiting, and rarely, peptic ulcers. Mechlorethamine must be used with extreme caution in patients with leukopenia, thrombocytopenia, or anemia caused by infiltration of bone marrow with malignant cells. In these patients, a good response to mechlorethamine therapy with disappearance of tumor from the bone marrow may be associated with improved bone marrow function; however, in the absence of good response or in patients who have received previous treatment with antineoplastic agents, hematopoiesis may be further compromised, resulting in more severe leukopenia, thrombocytopenia, anemia, and possibly death. Patients with chronic lymphocytic leukemia appear to be especially sensitive to the hematopoietic effects of mechlorethamine and should receive the drug with extreme caution, if at all. For more Drug Warnings (Complete) data for MECHLORETHAMINE HYDROCHLORIDE (17 total), please visit the HSDB record page. |
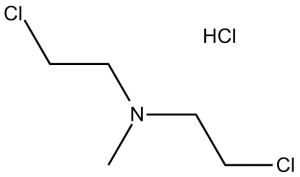
| 分子式 |
C5H11CL2N.HCL
|
|
|---|---|---|
| 分子量 |
192.51
|
|
| 精确质量 |
191.003
|
|
| 元素分析 |
C, 31.19; H, 6.28; Cl, 55.25; N, 7.28
|
|
| CAS号 |
55-86-7
|
|
| 相关CAS号 |
51-75-2 55-86-7 (HCl)
|
|
| PubChem CID |
5935
|
|
| 外观&性状 |
Hygroscopic leaflets from acetone or chloroform
White hygroscopic crystals White, crystalline, hygroscopic powder |
|
| 密度 |
1.106g/cm3
|
|
| 沸点 |
110.3ºC at 760 mmHg
|
|
| 熔点 |
108-111 °C(lit.)
|
|
| 闪点 |
20.5ºC
|
|
| 折射率 |
1.4689 (24ºC)
|
|
| LogP |
2.197
|
|
| tPSA |
3.24
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
1
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
9
|
|
| 分子复杂度/Complexity |
43.7
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C([H])([H])Cl.Cl[H]
|
|
| InChi Key |
QZIQJVCYUQZDIR-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C5H11Cl2N.ClH/c1-8(4-2-6)5-3-7;/h2-5H2,1H3;1H
|
|
| 化学名 |
2-chloro-N-(2-chloroethyl)-N-methylethanamine;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (10.80 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (10.80 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (10.80 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (519.45 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.1945 mL | 25.9727 mL | 51.9454 mL | |
| 5 mM | 1.0389 mL | 5.1945 mL | 10.3891 mL | |
| 10 mM | 0.5195 mL | 2.5973 mL | 5.1945 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06149247 | Recruiting | Drug: Mechlorethamine Topical Gel Drug: Hypericin |
Cutaneous T-Cell Lymphoma/Mycosis Fungoides |
Soligenix | December 5, 2023 | Phase 2 |
| NCT03380026 | Completed | Drug: Valchlor 0.016 % Topical Gel Drug: Triamcinolone |
Granulomatous Slack Skin Mycosis Fungoides |
Rochester Skin Lymphoma Medical Group, PLLC |
December 13, 2017 | Phase 2 |
| NCT00535470 | Completed | Drug: 0.04% Mechlorethamine gel | Mycosis Fungoides | Yaupon Therapeutics | July 2007 | Phase 2 |
| NCT00792467 | Completed | Drug: ITF2357 | Hodgkin's Lymphoma | Italfarmaco | February 2008 | Phase 1 Phase 2 |