| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
Progesterone receptor; Endogenous Metabolite
Phosphatidylinositol 3-Kinase (PI3K)/Akt/Nuclear Factor-kappaB (NF-κB) Pathway: Medroxyprogesterone acetate (MPA) activates this pathway to upregulate cyclin D1 [1] - Cell Adhesion Molecules (VCAM-1, ICAM-1): MPA upregulates their expression in endothelial cells [2] |
|---|---|
| 体外研究 (In Vitro) |
在类固醇剥夺的 HUVEC 中,醋酸甲羟孕酮(10 和 0.5 nM,48 小时)抑制 eNOS 表达 [2]。 Medroxyprogesteroneacetate(10和0.5nM,16小时)降低内皮粘附分子(VCAM-1和ICAM-1蛋白)的表达,从而防止白细胞粘附到人内皮细胞(类固醇剥夺的HUVEC)上[2]。在类固醇剥夺的 HUVEC 中,醋酸甲羟孕酮(10 和 0.5 nM,2 小时)可减少 NF-κB 核易位 [2]。
1. 人乳腺癌细胞增殖诱导([1]): 用MPA(10–1000 nM)处理T47D和MCF-7(PR阳性乳腺癌)细胞48小时,呈浓度依赖促进增殖。100 nM时,T47D细胞活力较对照升高35%,MCF-7升高30%(MTT实验)。蛋白质印迹法显示,100 nM MPA使cyclin D1蛋白水平在T47D中升高2.5倍,MCF-7中升高2倍;用PI3K抑制剂(LY294002,10 μM)或NF-κB抑制剂(PDTC,20 μM)处理后,MPA诱导的cyclin D1上调和增殖被阻断(活力仅较对照高10%)[1] 2. 单核细胞-内皮细胞相互作用增强([2]): 人脐静脉内皮细胞(HUVECs)用MPA(10–1000 nM)处理24小时,100 nM时VCAM-1 mRNA升高3倍,ICAM-1 mRNA升高2.5倍(实时PCR),对应蛋白水平分别升高2.8倍和2.2倍(蛋白质印迹法)。在流动条件(1 dyn/cm²)下,100 nM MPA使荧光标记的THP-1单核细胞与HUVECs的黏附率升高40%(荧光显微镜计数)[2] 3. 甲状腺癌细胞细胞因子下调([6]): 用MPA(1–10 μM)处理KTC-2(间变性甲状腺癌)细胞48小时,10 μM时IL-6分泌降低55%(ELISA),PTHrP mRNA降低60%(实时PCR)。MTT实验显示,10 μM MPA对细胞活力无显著影响(活力>90%)[6] 4. 无神经保护作用([5]): 大鼠原代海马神经元在谷氨酸损伤前用MPA(1–10 μM)处理,MPA未减少神经元死亡(乳酸脱氢酶释放与损伤对照无差异),也未上调神经保护蛋白(BDNF、Bcl-2)[5] |
| 体内研究 (In Vivo) |
大鼠灌胃给予 5 mg/kg 醋酸甲羟孕酮,观察到 AUC0-2535.9 ng·h/mL,t1/2 为 10.2 小时,Cmax 为 377.9 ng/mL [3]。在 14 天的过程中,口服醋酸甲羟孕酮(0.05-0.2 mg/kg/天)的大鼠显示,除肾上腺外的所有组织中别孕酮水平均有所增加,并且海马中的 β-END 水平发生了变化 [4]。
甲羟孕酮(27.7 μg/天,皮下注射)虽能增强血栓形成,但能抑制动脉血管血栓形成[3]。 MPA和MPA + e2治疗的动物表现出加重的血栓反应,表现为稳定闭塞的时间显著缩短。MPA的促血栓作用与ETP升高平行,而血小板活化不受影响。此外,MPA + E2减少了α -平滑肌肌动蛋白阳性细胞的数量,增加了斑块基质中的透明质酸。有趣的是,总斑块负担减少了MPA,但MPA + E2没有变化[3]。 结论和意义:长期服用MPA和MPA + E2可增加apoe缺陷小鼠动脉血栓形成,尽管MPA对动脉粥样硬化有抑制作用。凝血酶形成增加,平滑肌含量减少和非胶原斑块基质重塑可能参与促血栓作用。因此,MPA对动脉血栓形成和动脉粥样硬化的影响不同[3]。 甲羟孕酮醋酸酯(MPA)和MPA + e2治疗的动物表现出加重的血栓反应,表现为稳定闭塞的时间显著缩短。MPA的促血栓作用与ETP升高平行,而血小板活化不受影响。此外,MPA + E2减少了α -平滑肌肌动蛋白阳性细胞的数量,增加了斑块基质中的透明质酸。有趣的是,MPA降低了总斑块负担,但MPA + E2没有改变。 结论和意义:长期服用MPA和MPA + E2可增加apoe缺陷小鼠动脉血栓形成,尽管MPA对动脉粥样硬化有抑制作用。凝血酶形成增加,平滑肌含量减少和非胶原斑块基质重塑可能参与促血栓作用。因此,MPA对动脉血栓形成和动脉粥样硬化的影响是不同的。[4] 1. 小鼠血栓与动脉粥样硬化影响([3]): - 血栓模型:8–10周龄雄性C57BL/6小鼠皮下注射MPA(1、5、10 mg/kg/天),连续14天。10 mg/kg剂量使尾出血时间缩短30%,三氯化铁诱导的颈动脉血栓形成率升高40%。 - 动脉粥样硬化模型:12周龄ApoE⁻/⁻小鼠口服MPA(5 mg/kg/天),连续12周。主动脉根部斑块面积较对照增加25%,巨噬细胞浸润增多(CD68免疫组化)[3] 2. 大鼠无神经保护作用([5]): 10周龄雌性SD大鼠在海马缺血再灌注损伤前,皮下注射MPA(2 mg/kg/天),连续7天。MPA未减少梗死体积(TTC染色),也未改善认知功能(Morris水迷宫逃避潜伏期无缩短)[5] |
| 酶活实验 |
卵巢激素黄体酮在不同的神经变性实验模型中具有神经保护作用。在神经系统中,孕酮通过5 α还原酶代谢为5 α -二氢孕酮(DHP)。DHP随后通过3 -羟基类固醇脱氢酶催化的可逆反应还原为3 -,5 -四氢孕酮(THP)。[5]
醋酸甲羟孕酮(MPA)诱导乳腺癌细胞增殖的机制尚不清楚。本研究探讨了MPA影响孕激素受体(PR)阳性T47D人乳腺癌细胞中cyclin D1表达的机制。MPA (10 nM)处理48 h,细胞增殖率达到1.6倍。MPA诱导细胞周期蛋白D1表达(诱导率为3.3倍),选择性PR拮抗剂RU486阻断MPA诱导的细胞增殖和细胞周期蛋白D1表达(抑制率为23%)。MPA使转染PRB而不转染PRA的MCF-7细胞中cyclin D1的蛋白水平(诱导率为2.2倍)和启动子活性(诱导率为2.7倍)均升高。虽然MPA转录激活了cyclin D1的表达,但cyclin D1启动子没有孕激素应答元件相关序列。我们进一步研究了cyclin D1表达调控的机制。由于cyclin D1启动子包含三个假定的核因子- kappab (NFkappaB)结合基序,并且NFkappaB是Akt的底物,因此我们研究了磷脂酰肌醇3-激酶(PI3K)/Akt/NFkappaB级联对cyclin D1对MPA的反应的影响。MPA诱导Akt瞬时磷酸化(5 min诱导2.7倍),PI3K抑制剂(wortmannin)处理可减弱MPA诱导的cyclin D1表达上调(抑制40%)和细胞增殖(抑制40%)。MPA还诱导了NFkappaBalpha抑制剂(IkappaBalpha)的磷酸化(诱导率为2.3倍),而wortmannin处理可减弱MPA诱导的IkappaBalpha磷酸化(抑制率为60%)。用IkappaBalpha磷酸化抑制剂(BAY 11-7085)或特异性NFkappaB核易位抑制剂(SN-50)处理可以减弱mpa诱导的cyclin D1表达上调(分别抑制80%和50%)和细胞增殖(分别抑制55%和34%)。由于MPA诱导Akt的短暂磷酸化,且cyclin D1启动子不含孕酮响应元件相关序列,因此MPA通过PRB通过PI3K/Akt/NFkappaB级联上调cyclin D1表达诱导细胞增殖可能是非基因组机制[1]。 本文首次利用秀丽隐杆线虫(Cunninghamella elegans)、玫瑰毛霉(Trichothecium roseum)和铅毛霉(Mucor plumbeus)研究了甲氧孕酮(1)的真菌转化。得到的代谢物如下:6β,20-dihydroxymedroxyprogesterone(2), 12个β-hydroxymedroxyprogesterone(3), 6β,11β-dihydroxymedroxyprogesterone(4), 16β-hydroxymedroxyprogesterone(5), 11α,17-dihydroxy-6α-methylpregn-4-ene-3, 20-dione (6), 11-oxo-medroxyprogesterone(7) 6α-methyl-17α-hydroxypregn-1, 4-diene-3, 20-dione(8)和6β-hydroxymedroxyprogesterone(9), 15β-hydroxymedroxyprogesterone(10), 6α-methyl-17α,11β-dihydroxy-5α-pregnan-3, 20-dione(11), 11个β-hydroxymedroxyprogesterone(12)和11α,20-dihydroxymedroxyprogesterone(13)。在所有微生物转化产物中,新分离的生物转化产物13对SH-SY5Y细胞的增殖活性最强。化合物12、5、6、9、11和3对SH-SY5Y肿瘤细胞系也显示出一定程度的活性(活性由高到低)。从未报道过的生物转化产物2显示出对乙酰胆碱酯酶最有效的抑制活性。进行分子模拟研究是为了了解观察到的实验活动,也为了获得更多关于生物转化产物与酶之间的结合模式和相互作用的信息。[4] |
| 细胞实验 |
免疫荧光[2]
细胞类型:100 ng/ml LPS 处理的内皮细胞 测试浓度:10 和 0.5 nM 孵育时间:2h 实验结果:抑制NF-κB核转位。 在HUVECs中,通过实时PCR检测粘附分子mRNA水平。免疫细胞化学和酶联免疫吸附测定蛋白表达。为了模拟单核细胞粘附内皮细胞,我们使用了一个流动室系统来评估孕激素对U937单核细胞粘附HUVEC单层的影响。我们还研究了小干扰rna对粘附分子的抑制作用。[2] 从甲状腺癌复发伴甲状腺乳头状癌间变性的恶性胸腔积液中建立了一种新的甲状腺癌细胞系KTC-2。核型分析显示有109条染色体。皮下细胞注射在胸腺或严重联合免疫缺陷疾病(SCID)小鼠中产生小的退行性肿瘤。组织学检查显示间变性肿瘤细胞被突出的单核细胞包围。检测到甲状腺球蛋白、甲状腺转录因子-1和PAX-8的表达,但未检测到甲状腺过氧化物酶和促甲状腺激素(TSH)受体。生化分析显示分泌白介素(IL)-6、甲状旁腺激素相关蛋白(PTHrP)和粒细胞-巨噬细胞集落刺激因子。已知所有细胞因子均可诱导间变性甲状腺癌患者的副肿瘤综合征。我们之前的研究表明,醋酸甲羟孕酮(MPA)可降低人乳腺癌细胞IL-6和PTHrP的分泌。为了研究这些细胞因子的分泌调节机制,我们给KTC-2细胞注射MPA。MPA呈剂量依赖性降低IL-6和PTHrP的分泌及mRNA表达。雄激素受体和糖皮质激素受体(GR)均有表达,孕激素受体未见表达。地塞米松能降低IL-6和PTHrP的分泌,而双氢睾酮和孕酮不能。这些结果表明,MPA作为GR介导的糖皮质激素,可降低KTC-2细胞中IL-6和PTHrP的分泌。KTC-2细胞系可能是开发抗间变性甲状腺癌引起的副肿瘤综合征新策略的合适模型。[6] 1. 乳腺癌细胞实验([1]): - 细胞培养:T47D/MCF-7细胞接种于含10%胎牛血清的RPMI 1640培养基,96孔板(5×10³细胞/孔)或6孔板(2×10⁵细胞/孔)。 - 药物处理:贴壁24小时后,用MPA(10–1000 nM)单独或与PI3K抑制剂(LY294002,10 μM)/NF-κB抑制剂(PDTC,20 μM)共处理48小时。 - 检测: 1. 增殖:MTT实验(570 nm吸光度)计算活力; 2. 蛋白表达:蛋白质印迹法检测cyclin D1、p-Akt、p-NF-κB(以β-肌动蛋白为内参)[1] 2. 内皮-单核细胞相互作用实验([2]): - 细胞培养:HUVECs接种于24孔板(1×10⁵细胞/孔)培养至融合;THP-1单核细胞用CM-DiI荧光染料标记。 - 药物处理:HUVECs用MPA(10–1000 nM)处理24小时,加入THP-1细胞(5×10⁴细胞/孔),在流动条件(1 dyn/cm²)下孵育30分钟。 - 检测: 1. 黏附:荧光显微镜计数黏附的THP-1细胞; 2. 基因/蛋白:实时PCR检测VCAM-1/ICAM-1 mRNA,蛋白质印迹法检测对应蛋白[2] 3. 甲状腺癌细胞实验([6]): - 细胞培养:KTC-2细胞接种于含10%胎牛血清的DMEM培养基,6孔板(1×10⁵细胞/孔)。 - 药物处理:用MPA(1–10 μM)处理48小时。 - 检测: 1. 细胞因子分泌:ELISA检测培养上清中IL-6; 2. 基因表达:实时PCR检测PTHrP mRNA(以GAPDH为内参)[6] |
| 动物实验 |
Apolipoprotein E (ApoE)-/- mice were bilaterally ovariectomized and treated with placebo, MPA (27.7 microg day(-1)) and MPA + 17-beta-oestradiol (E2; 1.1 microg day(-1)) for 90 days, on a Western-type diet. Thrombotic response was measured in a photothrombosis model, platelet activation by fluorescence activated cell sorting (FACS) analysis (CD62P) and thrombin generation by the endogenous thrombin potential (ETP). Furthermore, aortic plaque burden and aortic root plaque composition were determined.[3]
Ovariectomy and Hormone Treatment[7] Rats were randomly assigned to one of five treatment groups: Sham (ovary-intact), OVX, OVX+PROG, OVX+Low MPA, and OVX+High MPA. Approximately two months before behavioral testing, all rats received OVX or sham surgery. All rats were anesthetized via isofluorene inhalation. Rats receiving OVX underwent bilateral dorsolateral incisions in the skin and peritoneum, and the ovaries and tips of uterine horns were ligatured and removed. Muscle and skin were then sutured. Rats receiving sham surgery underwent identical skin incision and suture. At the time of surgery, Alzet osmotic pumps (2ML4) containing either proplyene glycol (vehicle), progesterone (PROG; 21 mg dissolved in 2 mL propylene glycol), or MPA (low dose: 14 mg; high dose: 21 mg, dissolved in 2 mL propylene glycol) were implanted in the neck scruff. Hormone administration continued throughout behavior testing and sacrifice. Doses were based on prior research (Zhang, Fishman, and Huang, 1999), multiplied by a factor of 10 to account for the increased weight from the mouse to the rat. After surgery, rats received Rimadyl (5 mg/mL/kg) for pain and saline (2 mL) to prevent dehydration. Animals underwent pump reinsertion surgery every 31–32 days; behavioral testing began 66 days after the first pump insertion. Thus, hormone administration continued throughout behavior testing and sacrifice.[7] 1. Mouse Thrombosis/Atherosclerosis Protocol ([3]): - Thrombosis Model: 1. Animal Selection: 8–10 weeks old male C57BL/6 mice (n=6/group) randomized to control, 1, 5, 10 mg/kg MPA. 2. Drug Preparation: MPA dissolved in sesame oil (0.1, 0.5, 1 mg/mL). 3. Administration: Subcutaneous injection (0.1 mL/10 g body weight) once daily for 14 days. 4. Detection: Tail bleeding time measured; carotid thrombosis induced by ferric chloride, thrombus formation rate recorded. - Atherosclerosis Model: 1. Animal Selection: 12 weeks old ApoE⁻/⁻ mice (n=6/group) fed high-fat diet. 2. Drug Preparation: MPA suspended in 0.5% CMC + 0.1% Tween 80 (0.5 mg/mL). 3. Administration: Oral gavage (10 mL/kg) once daily for 12 weeks. 4. Detection: Aortic root sectioned for H&E staining (plaque area) and CD68 immunohistochemistry (macrophages) [3] 2. Rat Neuroprotection Protocol ([5]): - Animal Selection: 10 weeks old female Sprague-Dawley rats (n=5/group) randomized to control, injury, injury+MPA. - Drug Preparation: MPA dissolved in ethanol (5%) + normal saline (95%) (0.2 mg/mL). - Administration: Subcutaneous injection (10 mL/kg) once daily for 7 days (1st injection 24 hours before ischemia). - Detection: Hippocampal infarct volume measured via TTC staining; cognitive function assessed via Morris water maze (escape latency) [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption of oral medroxyprogesterone acetate (MPA) varies considerably between formulations. A 1000mg oral dose reaches an average Cmax of 145-315nmol/L while a 500mg oral dose reaches an average Cmax of 33-178nmol/L with a Tmax of 1-3 hours and a lag time of half an hour. The AUC of a 500mg oral dose of MPA was 543.4-1981.1nmol\L/h depending on formulation. Intramuscular MPA reaches a Cmax of 4.69±1.52nmol/L with a Tmax of 4.75±2.09 days and an AUC of 81.58±27.64days\nmol/L. Subcutaneous MPA reaches a Cmax of 3.83±1.56nmol/L with a T±max of 6.52±2.07 days and an AUC of 72.26±38.73days\nmol/L. However, the pharmacokinetics of MPA may also vary depending on injection site. The majority of medroxyprogesterone acetate (MPA) is eliminated in the urine as glucuronide conjugates and a minority of sulphate conjugates. Glucuronide conjugates are also detected in the feces. Determining the exact ratio of metabolites and parent compound eliminated in the urine and feces is difficult as the metabolite profile in the urine is not significantly different and radio labelling studies are not readily available. The volume of distribution of medroxyprogesterone acetate is 20±3L. The mean clearance of medroxyprogesterone acetate (MPA) is 1668±146L/day or 21.9±4.3L/kg/day. Due to the high inter patient variability in MPA pharmacokinetics, clearance has been reported to be 1600-4000L/day. Metabolism / Metabolites Medroxyprogesterone acetate undergoes beta hydroxylation to form the metabolites 6-beta (M-2), 2-beta (M-4), and 1-beta-hydroxymedroxyprogesterone acetate (M-3). M-2 and M-4 are further metabolized to 2-beta,6-beta-dihydroxymedroxyprogesterone (M-1). M-3 is further metabolized to 1,2-dehydromedroxyprogesterone acetate (M-5). Medroxyprogesterone Acetate has known human metabolites that include M-3, Medroxyprogesterone Acetate, and M-2. Hepatic. Route of Elimination: Following oral dosing, MPA is extensively metabolized in the liver via hydroxylation, with subsequent conjugation and elimination in the urine. Most MPA metabolites are excreted in the urine as glucuronide conjugates with only minor amounts excreted as sulfates. Half Life: 50 days Biological Half-Life Oral medroxyprogesterone acetate (MPA) has an absorption half life of 15-30min and a biological half life of 40-60 hours. Intramuscular MPA has an absorption half life of 0.86±0.30 days and an elimination half life of 24.03±21.74 days. Subcutaneous MPA has an absorption half life of 1.05±0.56 days and an elimination half life of 30.90±15.11 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Progestins diffuse freely into target cells in the female reproductive tract, mammary gland, hypothalamus, and the pituitary and bind to the progesterone receptor. Once bound to the receptor, progestins slow the frequency of release of gonadotropin releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH surge. 1. In Vitro Toxicity: - MPA (10 μM) showed no cytotoxicity to KTC-2 cells (viability >90%, MTT assay) [6] - MPA (1–10 μM) did not induce neuronal necrosis (lactate dehydrogenase release <15% vs. control) [5] 2. In Vivo Toxicity: - Mice treated with MPA (1–10 mg/kg/day, 14–84 days) showed no changes in ALT/AST, BUN, or body weight [3] - Rats treated with MPA (2 mg/kg/day, 7 days) had no liver/kidney damage (serum biochemistry normal) [5] |
| 参考文献 |
[1]. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology. 2005 Nov;146(11):4917-25.
[2]. Medroxyprogesterone acetate enhances monocyte-endothelial interaction under flow conditions by stimulating the expression of cell adhesion molecules. J Clin Endocrinol Metab. 2014 Jun;99(6):2188-97. [3]. Differential effects of medroxyprogesterone acetate on thrombosis and atherosclerosis in mice. Br J Pharmacol. 2009 Dec;158(8):1951-60. [4]. Medroxyprogesterone derivatives from microbial transformation as anti-proliferative agents and acetylcholineterase inhibitors (combined in vitro and in silico approaches). Steroids. 2020 Dec;164:108735. [5]. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol.2006 Aug;66(9):916-28; [6]. Medroxyprogesterone acetate decreases secretion of interleukin-6 and parathyroid hormone-related protein in a new anaplastic thyroid cancer cell line, KTC-2. Thyroid.2003;13(3):249-58; [7]. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem.2010;93(3):444-53. |
| 其他信息 |
Medroxyprogesterone Acetate can cause cancer according to The World Health Organization's International Agency for Research on Cancer (IARC). It can cause developmental toxicity according to state or federal government labeling requirements.
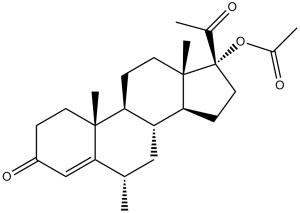
Medroxyprogesterone acetate is an odorless white to off-white microcrystalline powder. (NTP, 1992) Medroxyprogesterone acetate is an acetate ester resulting from the formal condensation of the 17alpha-hydroxy group of medroxyprogesterone with the carboxy group of acetic acid. A widely used progestin in menopausal hormone therapy and in progestogen-only birth control. It has a role as a progestin, an androgen, a female contraceptive drug, a synthetic oral contraceptive, an adjuvant, an inhibitor, an antioxidant and an antineoplastic agent. It is a steroid ester, an acetate ester, a 20-oxo steroid, a 3-oxo-Delta(4) steroid and a corticosteroid. It is functionally related to a medroxyprogesterone. Medroxyprogesterone acetate (MPA) is a [progesterone] derivative that is more resistant to metabolism for improved pharmacokinetic properties. MPA can be use to treat secondary amenorrhea, endometrial hyperplasia, abnormal uterine bleeding, osteoporosis, vasomotor symptoms in menopause, vulvar and vaginal atrophy, prevent pregnancy, manage pain in endometriosis, prevent pregnancy, and is also used in palliative care for endometrial and renal carcinoma. Medroxyprogesterone acetate was granted FDA approval on 18 June 1959. Medroxyprogesterone Acetate is a synthetic, acetate derivative of the sex hormone progesterone. Medroxyprogesterone 17-acetate (NCI04) Medroxyprogesterone acetate (INN, USAN, BAN), also known as 17‘±-hydroxy-6‘±-methylprogesterone acetate, and commonly abbreviated as MPA, is a steroidal progestin, a synthetic variant of the human hormone progesterone. It is used as a contraceptive, in hormone replacement therapy and for the treatment of endometriosis as well as several other indications. MPA is a more potent derivative of its parent compound medroxyprogesterone (MP). While medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate, what is normally being administered is MPA and not MP. A synthetic progestin that is derived from 17-hydroxyprogesterone. It is a long-acting contraceptive that is effective both orally or by intramuscular injection and has also been used to treat breast and endometrial neoplasms. Drug Indication Medroxyprogesterone acetate (MPA) oral tablets are indicated to treat secondary amenorrhea, reduce the incidence of endometrial hyperplasia in postmenopausal women, and to treat abnormal uterine bleeding due to hormonal imbalance, not organic pathology. Oral tablets containing MPA and conjugated estrogens are indicated to prevent postmenopausal osteoporosis and to treat moderate to severe menopausal symptoms such as vasomotor symptoms, vulvar atrophy, and vaginal atrophy. Subcutaneous MPA is indicated to prevent pregnancy and manage pain associated with endometriosis. Intramuscular MPA is indicated to prevent pregnancy, and at higher concentrations for palliative treatment of endometrial or renal carcinoma. FDA Label Mechanism of Action Medroxyprogesterone acetate (MPA) inhibits the production of gonadotropin, preventing follicular maturation and ovulation, which is responsible for it’s ability to prevent pregnancy. This action also thins the endometrium. MPA reduces nuclear estrogen receptors and DNA synthesis in epithelial cells of the endometrium. MPA can also induce p53 dependant apoptosis in certain cancer cell lines, and inhibit GABA-A receptors. Pharmacodynamics Medroxyprogesterone acetate (MPA) inhibits gonadotropin production, reduces nuclear estrogen receptors and DNA synthesis in epithelial cells of the endometrium, and induces p53 dependant apoptosis in cancer cell lines. MPA oral tablets have a half life of 40-60 hours and other formulations can have half lives that are considerably longer, so the duration of action is long. The therapeutic window is wide as patients may take doses ranging from 5mg orally daily to 1000mg as a depo injection weekly. Long term use of MPA is associated with a reduction in bone density and patients who taking MPA during adolescence may have lower peak bone mass than untreated patients, which can also increase the risk of osteoporosis and fractures in the future. 1. Drug Background ([1][3]): Medroxyprogesterone acetate is a synthetic progestin widely used in contraception, hormone replacement therapy, and treatment of hormone-sensitive cancers (e.g., breast, endometrial cancer) [1][3] 2. Mechanism of Action ([1][2][6]): - In breast cancer cells: Activates PI3K/Akt/NF-κB pathway to upregulate cyclin D1, promoting cell proliferation [1] - In endothelial cells: Upregulates VCAM-1/ICAM-1 to enhance monocyte adhesion, contributing to vascular inflammation [2] - In thyroid cancer cells: Downregulates IL-6 and PTHrP secretion, potentially inhibiting tumor progression [6] 3. Limitation ([5]): Unlike progesterone (endogenous progestin), MPA lacks neuroprotective effects due to its inability to be metabolized into neuroactive reduced metabolites [5] |
| 分子式 |
C24H34O4
|
|
|---|---|---|
| 分子量 |
386.52
|
|
| 精确质量 |
386.245
|
|
| 元素分析 |
, 74.58; H, 8.87; O, 16.56
|
|
| CAS号 |
71-58-9
|
|
| 相关CAS号 |
Medroxyprogesterone acetate;71-58-9; 520-85-4
|
|
| PubChem CID |
6279
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.1±0.1 g/cm3
|
|
| 沸点 |
496.4±45.0 °C at 760 mmHg
|
|
| 熔点 |
206-207 °C(lit.)
|
|
| 闪点 |
213.2±28.8 °C
|
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
|
| 折射率 |
1.539
|
|
| LogP |
4.11
|
|
| tPSA |
60.44
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
767
|
|
| 定义原子立体中心数目 |
7
|
|
| SMILES |
C[C@H]1C[C@@H]2[C@H](CC[C@]3([C@H]2CC[C@@]3(C(=O)C)OC(=O)C)C)[C@@]4(C1=CC(=O)CC4)C
|
|
| InChi Key |
PSGAAPLEWMOORI-PEINSRQWSA-N
|
|
| InChi Code |
InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1
|
|
| 化学名 |
(6S,8R,9S,10R,13S,14S,17R)-17-acetyl-6,10,13-trimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl acetate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (2.59 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (2.59 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (2.59 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5872 mL | 12.9359 mL | 25.8719 mL | |
| 5 mM | 0.5174 mL | 2.5872 mL | 5.1744 mL | |
| 10 mM | 0.2587 mL | 1.2936 mL | 2.5872 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。