| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
OX1 ( IC50 = 1789 nM ); OX2 ( IC50 = 18 nM ); OX1 ( Ki = 1584 nM ); OX2 ( Ki = 0.5 nM )
|
|
|---|---|---|
| 体外研究 (In Vitro) |
体外活性:MK-1064是一种新型、强效、选择性、口服生物可利用的Orexin OX2受体拮抗剂,具有用于治疗失眠的潜力。食欲素神经肽通过食欲素受体(OX1R、OX2R)调节睡眠/觉醒; OX2R 是唤醒促进的主要调节因子。 MK-1064 是一种 OX2R 单一拮抗剂。临床前,MK-1064 可以促进睡眠,并增加大鼠的快速眼动 (REM) 和非快速眼动 (NREM) 睡眠,OX2R 占用率高于双食欲素受体拮抗剂观察到的范围。
|
|
| 体内研究 (In Vivo) |
与双重拮抗剂类似,MK-1064 可以增加狗的 NREM 和 REM 睡眠,而不诱发猝倒。两项针对健康人类受试者的 I 期研究评估了 MK-1064 的安全性、耐受性、药代动力学和睡眠促进作用,并证明主观嗜睡(通过卡罗林斯卡嗜睡量表和视觉模拟量表测量)和睡眠(通过多导睡眠图)呈剂量依赖性增加,包括增加 REM 和 NREM 睡眠。因此,选择性 OX2R 拮抗作用足以促进跨物种的 REM 和 NREM 睡眠,类似于双重食欲素受体拮抗作用。 MK-1064 可促进睡眠并增加大鼠的快速眼动 (REM) 和非快速眼动 (NREM) 睡眠,OX2R 占用率高于双食欲素受体拮抗剂观察到的范围。 MK-1064 可以增加狗的 NREM 和 REM 睡眠,而不诱发猝倒。动物给药参考值为30mg/kg。
|
|
| 动物实验 |
|
|
| 参考文献 |
|
|
| 其他信息 |
MK-1064 is under investigation in clinical trial NCT02549014 (A Single Dose Study of the Safety, Pharmacokinetics and Pharmacodynamics of MK-1064 (MK-1064-001)).
The field of small-molecule orexin antagonist research has evolved rapidly in the last 15 years from the discovery of the orexin peptides to clinical proof-of-concept for the treatment of insomnia. Clinical programs have focused on the development of antagonists that reversibly block the action of endogenous peptides at both the orexin 1 and orexin 2 receptors (OX1R and OX2R), termed dual orexin receptor antagonists (DORAs), affording late-stage development candidates including Merck’s suvorexant (new drug application filed 2012). Full characterization of the pharmacology associated with antagonism of either OX1R or OX2R alone has been hampered by the dearth of suitable subtype-selective, orally bioavailable ligands. Herein, we report the development of a selective orexin 2 antagonist (2-SORA) series to afford a potent, orally bioavailable 2-SORA ligand. Several challenging medicinal chemistry issues were identified and overcome during the development of these 2,5-disubstituted nicotinamides, including reversible CYP inhibition, physiochemical properties, P-glycoprotein efflux and bioactivation. This article highlights structural modifications the team utilized to drive compound design, as well as in vivo characterization of our 2-SORA clinical candidate, 5′′-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2′:5′,3′′-terpyridine-3′-carboxamide (MK-1064), in mouse, rat, dog, and rhesus sleep models.[1] Orexin neuropeptides regulate sleep/wake through orexin receptors (OX1R, OX2R); OX2R is the predominant mediator of arousal promotion. The potential for single OX2R antagonism to effectively promote sleep has yet to be demonstrated in humans. MK-1064 is an OX2R-single antagonist. Preclinically, MK-1064 promotes sleep and increases both rapid eye movement (REM) and non-REM (NREM) sleep in rats at OX2R occupancies higher than the range observed for dual orexin receptor antagonists. Similar to dual antagonists, MK-1064 increases NREM and REM sleep in dogs without inducing cataplexy. Two Phase I studies in healthy human subjects evaluated safety, tolerability, pharmacokinetics and sleep-promoting effects of MK-1064, and demonstrated dose-dependent increases in subjective somnolence (via Karolinska Sleepiness Scale and Visual Analogue Scale measures) and sleep (via polysomnography), including increased REM and NREM sleep. Thus, selective OX2R antagonism is sufficient to promote REM and NREM sleep across species, similarly to that seen with dual orexin receptor antagonism.[2] Orexins are hypothalamic neuropeptides that have a documented role in mediating the acute stress response. However, their role in habituation to repeated stress, and the role of orexin receptors (OX1R and OX2R) in the stress response, has yet to be defined. Orexin neuronal activation and levels in the cerebrospinal fluid (CSF) were found to be stimulated with acute restraint, but were significantly reduced by day five of repeated restraint. As certain disease states such as panic disorder are associated with increased central orexin levels and failure to habituate to repeated stress, the effect of activating orexin signaling via Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) on the hypothalamic-pituitary-adrenal (HPA) response was evaluated after repeated restraint. While vehicle-treated rats displayed habituation of Adrenocorticotropic Hormone (ACTH) from day 1 to day 5 of restraint, stimulating orexins did not further increase ACTH beyond vehicle levels for either acute or repeated restraint. We delineated the roles of orexin receptors in acute and repeated stress using a selective OX2R antagonist (MK-1064). Pretreatment with MK-1064 reduced day 1 ACTH levels, but did not allow further habituation on day 5 compared with vehicle-treated rats, indicating that endogenous OX2R activity plays a role in acute stress, but not in habituation to repeated stress. However, in restrained rats with further stimulated orexins by DREADDs, MK-1064 decreased ACTH levels on day 5. Collectively, these results indicate that the OX2R plays a role in acute stress, and can prevent habituation to repeated stress under conditions of high orexin release.[3] |
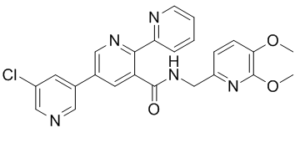
| 分子式 |
C24H20CLN5O3
|
|
|---|---|---|
| 分子量 |
461.91
|
|
| 精确质量 |
461.125
|
|
| 元素分析 |
C, 62.41; H, 4.36; Cl, 7.67; N, 15.16; O, 10.39
|
|
| CAS号 |
1207253-08-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
44633765
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
662.4±55.0 °C at 760 mmHg
|
|
| 闪点 |
354.4±31.5 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.619
|
|
| LogP |
3.04
|
|
| tPSA |
99.1
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
629
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1=C([H])N=C([H])C(=C1[H])C1C([H])=NC(C2=C([H])C([H])=C([H])C([H])=N2)=C(C(N([H])C([H])([H])C2C([H])=C([H])C(=C(N=2)OC([H])([H])[H])OC([H])([H])[H])=O)C=1[H]
|
|
| InChi Key |
CKTWQGHVNRYNCM-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31)
|
|
| 化学名 |
5-(5-chloropyridin-3-yl)-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2-pyridin-2-ylpyridine-3-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1649 mL | 10.8246 mL | 21.6492 mL | |
| 5 mM | 0.4330 mL | 2.1649 mL | 4.3298 mL | |
| 10 mM | 0.2165 mL | 1.0825 mL | 2.1649 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02549014 | Completed | Drug: MK-1064 Drug: Placebo |
Pharmacokinetics | Merck Sharp & Dohme LLC | July 6, 2009 | Phase 1 |
| NCT02549027 | Completed | Drug: MK-1064 Drug: Placebo Drug: MK-6096 |
Polysomnography | Merck Sharp & Dohme LLC | November 6, 2009 | Phase 1 |
 MK-1064 dose-dependently promotes somnolence and attenuates arousal in healthy human subjects.Sci Rep.2016 Jun 3;6:27147. |
|---|
 MK-1064 promotes sleep in healthy subjects.Sci Rep.2016 Jun 3;6:27147. |
 Sleep effects of MK-1064 30 mg/kg are OX2R-dependent.
Enrolment information for Phase I studies in healthy human volunteers.Sci Rep.2016 Jun 3;6:27147. |
 MK-1064 dose-dependently promotes both NREM and REM sleep during the active phase in rats.Sci Rep.2016 Jun 3;6:27147. |
|---|
 MK-1064 requires higher OX2R occupancies to promote sleep relative to DORA-12 in rats.Sci Rep.2016 Jun 3;6:27147. |
 MK-1064 effectively promotes somnolence but not cataplexy in canines.Sci Rep.2016 Jun 3;6:27147. |