| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
TSHR ( IC50 = 2.1 μM ); LHR ( IC50 > 30 μM ); FSHR ( IC50 > 30 μM )
ML224 is a selective antagonist of the thyroid-stimulating hormone receptor (TSHR); Ki value for human TSHR is 230 nM (determined by [125I]TSH competitive binding assay), and IC50 value for inhibiting TSH-induced cAMP accumulation in CHO-hTSHR cells is 180 nM. It exhibits >100-fold selectivity over other glycoprotein hormone receptors (e.g., luteinizing hormone receptor, follicle-stimulating hormone receptor) and unrelated GPCRs (e.g., β2-adrenergic receptor) at concentrations up to 10 μM. [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:ML224,也称为 NCGC00242364 和 ANTAG3,是甲状腺刺激激素受体 (TSHR) 的选择性反向激动剂。它抑制 TSH 刺激的 cAMP 产生,IC50 为 2.3 μM。 ANTAG3 对 TSHR 抑制具有选择性,TSHR 的半最大抑制剂量为 2.1 μM,LH 和 FSH 受体的半最大抑制剂量大于 30 μM。在接受 TRH 治疗的小鼠中,ANTAG3 使血清游离 T4 降低 44%,使钠碘协同转运蛋白和甲状腺过氧化物酶的 mRNA 分别降低 75% 和 83%。在给予 M22 的小鼠中,ANTAG3 使血清游离 T4 降低了 38%,并将钠碘协同转运蛋白和甲状腺过氧化物酶的 mRNA 分别降低了 73% 和 40%。总之,我们开发了一种在小鼠体内有效的选择性 TSHR 拮抗剂。这是小分子 TSHR 拮抗剂在体内具有活性的首次报道,可能会开发出治疗格雷夫斯病的药物。激酶测定:ML224,也称为 NCGC00242364 和 ANTAG3,是甲状腺刺激激素受体 (TSH)R) 的选择性反向激动剂。它抑制 TSH 刺激的 cAMP 产生,IC50 为 2.3 μM。 ANTAG3 对 TSHR 抑制具有选择性,TSHR 的半最大抑制剂量为 2.1 μM,LH 和 FSH 受体的半最大抑制剂量大于 30 μM。细胞测定:
1. TSHR竞争性结合与拮抗作用:ML224竞争性结合人TSHR,置换[125I]TSH,Ki=230 nM。在稳定表达人TSHR的CHO细胞中,剂量依赖性抑制TSH(10 mU/mL)诱导的cAMP积累,IC50=180 nM;1 μM浓度下,cAMP生成较溶媒对照组减少82%,但对毛喉素(forskolin)诱导的cAMP积累无显著抑制(表明不直接影响腺苷酸环化酶活性)。[1] 2. 高受体选择性:ML224(10 μM)对黄体生成素受体(LHR)、促卵泡激素受体(FSHR)及β2肾上腺素受体无显著结合或功能拮抗作用,对这些受体的抑制率<10%,证实对TSHR的高选择性。[1] 3. 抑制甲状腺激素合成相关基因表达:在人甲状腺滤泡上皮原代细胞中,ML224(200 nM)抑制TSH诱导的甲状腺过氧化物酶(TPO)和钠碘同向转运体(NIS)mRNA表达上调,分别抑制55%和61%(实时PCR检测)。[1] |
| 体内研究 (In Vivo) |
在接受 TRH 治疗的小鼠中,ANTAG3 使血清游离 T4 降低 44%,使钠碘协同转运蛋白和甲状腺过氧化物酶的 mRNA 分别降低 75% 和 83%。在给予 M22 的小鼠中,ANTAG3 使血清游离 T4 降低了 38%,钠碘协同转运蛋白和甲状腺过氧化物酶的 mRNA 分别降低了 73% 和 40%
1. 抑制雌性C57BL/6小鼠甲状腺功能:[1] - 6-8周龄雌性C57BL/6小鼠,每日一次口服给予ML224 10、30或100 mg/kg,持续7天。 - ML224剂量依赖性改变血清甲状腺激素水平:100 mg/kg剂量下,血清游离T4(fT4)水平较溶媒对照组降低42%,血清TSH水平升高2.8倍(符合负反馈调节机制),血清游离T3(fT3)水平无显著变化。 - 甲状腺重量:100 mg/kg组小鼠甲状腺重量较溶媒对照组减轻18%,体重无显著变化。 - 组织学分析:ML224处理组(100 mg/kg)甲状腺滤泡胶质含量减少,滤泡上皮细胞轻度扁平化,与甲状腺激素合成受抑一致。 2. 对雄性小鼠甲状腺功能无影响:雄性C57BL/6小鼠口服ML224 100 mg/kg(每日一次,持续7天),血清TSH、fT3、fT4水平无显著变化,表明其药效具有性别特异性。[1] |
| 酶活实验 |
ML224,也称为 NCGC00242364 和 ANTAG3,是甲状腺刺激激素受体 (TSH)R) 的选择性反向激动剂。它的 IC50 为 2.3 μM,可抑制 TSH 刺激的 cAMP 产生。 TSHR 抑制是 ANTAG3 选择性的唯一途径,TSHR 的半数最大抑制剂量为 2.1 μM,LH 和 FSH 受体的半数最大抑制剂量大于 30 μM。
1. TSHR竞争性结合实验(放射性配体结合法):[1] 从稳定表达人TSHR的CHO细胞中分离膜制剂,反应体系包含膜蛋白、[125I]标记TSH(示踪配体)及系列浓度的ML224。25°C孵育120分钟后,真空过滤玻璃纤维滤膜去除未结合配体,冰浴实验缓冲液洗涤滤膜,γ计数器测定放射性强度。通过置换曲线非线性回归分析,结合Cheng-Prusoff方程校正示踪配体浓度,计算Ki值。 2. TSHR功能拮抗cAMP积累实验:[1] CHO-hTSHR细胞接种于96孔板,孵育过夜后用系列浓度的ML224(10 nM–10 μM)预处理30分钟,加入TSH(10 mU/mL)刺激60分钟。采用均相时间分辨荧光(HTRF)cAMP检测试剂盒测定cAMP水平,基于相对于溶媒对照组的cAMP抑制量效曲线,确定IC50值。[1] |
| 细胞实验 |
细胞系:人胚肾 293 细胞(稳定表达 TSHR、LHR 或 FSHR)
浓度:0.001-100 µM 孵育时间:20 分钟 结果:显示牛 TSH 刺激的 IC50(1.8) nM) 为 2.1 µM。显示在 30 µM 时,LH (1 nM) 对 LH 和 FSH 刺激的抑制低于 15%,对 FSH (1 nM) 的抑制低于 30%。 1. 人甲状腺滤泡上皮原代细胞培养及基因表达实验:[1] 人甲状腺组织样本经胶原酶消化获得单个滤泡上皮细胞,甲状腺细胞培养基中培养48小时。细胞用ML224(50–200 nM)预处理1小时,再用TSH(5 mU/mL)刺激24小时。提取总RNA并逆转录为cDNA,实时PCR定量TPO和NIS mRNA表达水平,以GAPDH为内参基因,2^(-ΔΔCt)法计算相对表达量。 2. CHO-hTSHR细胞cAMP抑制实验:[1] 稳定表达人TSHR的CHO细胞以1×10⁴个/孔接种于96孔板,培养24小时后用无血清培养基洗涤,加入不同浓度的ML224(10 nM–10 μM)孵育30分钟。加入TSH(10 mU/mL)诱导cAMP生成,继续孵育60分钟后,HTRF试剂盒检测cAMP水平,计算相对于TSH刺激溶媒对照组的抑制率。[1] |
| 动物实验 |
Female BALB/c mice (8 to 13-week-old; ~18.7 g)
Dosage: 2 mg/mice Intraperitoneal injection via osmotic pump; single daily for 3 days 1. Thyroid function inhibition assay in female/male C57BL/6 mice: [1] - Animals: Female and male C57BL/6 mice (6-8 weeks old) were housed under a 12-hour light/dark cycle with free access to food and water. - Grouping and drug administration: Female mice were randomly divided into 4 groups (n=6 per group): vehicle control, ML224 10 mg/kg, 30 mg/kg, 100 mg/kg. Male mice were divided into 2 groups (n=6 per group): vehicle control and ML224 100 mg/kg. ML224 was dissolved in a solvent mixture of DMSO:PEG400:PBS (1:4:5, v/v/v) and administered via oral gavage once daily for 7 days. Vehicle control received the same volume of solvent. - Sample collection: Twenty-four hours after the last dose, mice were anesthetized, and blood was collected via retro-orbital puncture. Serum was separated by centrifugation and stored at -80°C for hormone analysis. Thyroid glands were excised, blotted dry, weighed, and fixed in 10% formalin for histological analysis. - Hormone detection: Serum TSH, fT3, and fT4 levels were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits. - Histological analysis: Formalin-fixed thyroid tissues were paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Follicle structure, colloid content, and epithelial cell morphology were observed under a light microscope. [1] |
| 参考文献 | |
| 其他信息 |
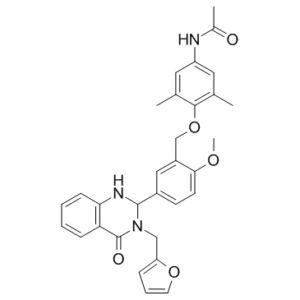
N-[4-[[5-[3-(2-furanylmethyl)-4-oxo-1,2-dihydroquinazolin-2-yl]-2-methoxyphenyl]methoxy]-3,5-dimethylphenyl]acetamide is a member of quinazolines.
1. Background: The thyroid-stimulating hormone receptor (TSHR) is a G protein-coupled receptor (GPCR) that mediates the biological effects of TSH, regulating thyroid hormone synthesis, secretion, and thyroid gland growth. Aberrant activation of TSHR is associated with thyroid disorders such as Graves' disease and toxic thyroid nodules. [1] 2. Mechanism of action: ML224 acts as a competitive antagonist of TSHR, binding to the extracellular domain of TSHR and preventing TSH from interacting with the receptor. This inhibits downstream cAMP signaling pathway activation, thereby suppressing thyroid hormone synthesis and secretion, and reducing thyroid gland hyperplasia. [1] 3. Gender-specific efficacy: ML224 exhibits significant thyroid function inhibition in female mice but no effect in male mice, possibly due to gender differences in TSHR expression or thyroid hormone regulatory pathways. [1] 4. Therapeutic potential: Preclinical data demonstrate that ML224 is a selective, orally active TSHR antagonist with potential applications in the treatment of TSHR-overactivated thyroid disorders (e.g., Graves' disease). However, further studies are needed to validate its efficacy in human patients and explore the underlying mechanism of gender specificity. [1] |
| 分子式 |
C31H31N3O5
|
|
|---|---|---|
| 分子量 |
525.59
|
|
| 精确质量 |
525.226
|
|
| 元素分析 |
C, 70.84; H, 5.95; N, 7.99; O, 15.22
|
|
| CAS号 |
1338824-21-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
50897809
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
752.3±60.0 °C at 760 mmHg
|
|
| 闪点 |
408.8±32.9 °C
|
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
|
| 折射率 |
1.626
|
|
| LogP |
2.92
|
|
| tPSA |
93Ų
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
829
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CC(NC1=CC(C)=C(OCC2=CC(C(N3CC4=CC=CO4)NC5=C(C=CC=C5)C3=O)=CC=C2OC)C(C)=C1)=O
|
|
| InChi Key |
BFTSWGYWHRJVNI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C31H31N3O5/c1-19-14-24(32-21(3)35)15-20(2)29(19)39-18-23-16-22(11-12-28(23)37-4)30-33-27-10-6-5-9-26(27)31(36)34(30)17-25-8-7-13-38-25/h5-16,30,33H,17-18H2,1-4H3,(H,32,35)
|
|
| 化学名 |
N-[4-[[5-[3-(furan-2-ylmethyl)-4-oxo-1,2-dihydroquinazolin-2-yl]-2-methoxyphenyl]methoxy]-3,5-dimethylphenyl]acetamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.03.00
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.76 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.76 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9026 mL | 9.5131 mL | 19.0262 mL | |
| 5 mM | 0.3805 mL | 1.9026 mL | 3.8052 mL | |
| 10 mM | 0.1903 mL | 0.9513 mL | 1.9026 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
ANTAG3 structure and selectivity.Endocrinology.2014 Jan;155(1):310-4. |
|---|
 ANTAG3 lowers serum FT4 levels and thyroidal mRNAs for TPO and NIS in mice continuously stimulated by TRH. A, TRH (2.4 μg/d for 3 d) was administered ip via osmotic pump with or without ANTAG3 (2 mg/d). The animals were euthanized on day 4. B, Serum FT4 levels. C, mRNA levels of TPO and NIS in thyroid gland lysates.Endocrinology.2014 Jan;155(1):310-4. |
 ANTAG3 lowers serum FT4 levels and thyroidal mRNAs for TPO and NIS in mice stimulated by a single injection of M22. A, T3(5 μg/d for 4 d) was administered by daily ip injection, and ANTAG3 was given for 3 days (2 mg/d) via an osmotic pump. On day 4, the animals were given an ip injection of vehicle or ANTAG3 (2 mg) and 4 hours later an ip injection of M22 (0.5 μg) or vehicle.Endocrinology.2014 Jan;155(1):310-4. |