| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Raf kinase
|
|---|---|
| 体外研究 (In Vitro) |
MLN2480 在 BRAF 突变体和一些 RAS 突变体临床前癌症模型体内耐受的浓度下抑制 MAPK 通路信号传导[1]。在非常低的浓度下,它会激活磷酸化 MEK,而在较高浓度下,它会抑制同样的活性。研究发现,MLN-2480 的抑制作用在模型和遗传背景之间存在差异[2]。 MLN2480 和 TAK-733(一种在研变构 MEK 激酶抑制剂)的药物组合在细胞增殖测定中的体外测试显示出协同活性。此外,蛋白质印迹分析显示 MLN2480 如何逆转 MEK 响应 TAK-733 的反馈激活,从而导致更协调的 MAPK 通路抑制。 PRAK 仅被 MLN-2480 微弱抑制 [1][2]。
Tovorafenib(MLN2480;BIIB-024;BSK1369;DAY-101;TAK-580;AMG-2112819)与naporafenib针对不同RAF亚型的效力比较[7] 为了更好地了解托vorafenib和naporafenib的RAF选择性,我们使用我们的TR-FRET实验测量了它们对上述RAF复合物的抑制作用。BRAFWT、BRAFV600E和CRAFSSDD在1 nM的浓度下检测,而CRAFWT和ARAFSSDD由于酶活性较低,分别在4 nM和10 nM的浓度下检测。ATP浓度为200 μM, WT MEK1底物浓度为250 nM。测量的IC50值和计算的Ki值如表1所示,其代表性的浓度-响应曲线如图2所示。[7] Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819)和naporafenib是最有效的CRAF抑制剂,对WT CRAF激酶的IC50值分别为94.2 nM和3.7 nM。对CRAFSSDD突变体的效价与对CRAFWT的效价基本相同(表1)。两种药物对BRAFWT和BRAFV600E的效价均为中等(托vorafenib对BRAFWT的效价为633 nM, naporafenib对BRAFWT的效价为13.4 nM),对ARAFSSDD的抑制作用弱得多。即使在10 μM的浓度下,Tovorafenib也不能完全抑制ARAFSSDD,这是我们在本实验中可以达到的最高抑制剂浓度。虽然tovorafenib和naporafenib在RAF亚型中共享效力趋势,但naporafenib对每种酶的效力至少比tovorafenib强一个数量级。先前关于naporafenib对纯化ARAF、BRAF和CRAF活性的研究报告的相对效力与我们观察到的相似,但IC50值明显较低(对CRAF为0.07 nM)(42)。本研究未提供反应条件,因此无法与我们的结果进行有意义的比较。[7] Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819)和naporafenib对CRAF和WT BRAF的抑制表明,这些RAF二聚体的抑制具有正协同性(图2)。使用四参数模型拟合这些曲线,允许可变Hill斜率,Hill斜率范围为- 2.6至- 3.2(表1)。这些值表明,tovorafenib和naporafenib对BRAF和CRAF二聚体的抑制具有显著的正协同性;也就是说,抑制剂与一个原聚体活性位点的结合增加了抑制剂与RAF二聚体中第二个原聚体结合的亲和力。我们在ARAFSSDD和BRAFV600E中都没有观察到这种效应,BRAFV600E在这个实验中是单体的(表1)。 |
| 体内研究 (In Vivo) |
MLN2480 在胰腺癌、肺癌、结肠癌和黑色素瘤的异种移植模型中表现出体内抗肿瘤活性[3]。 MLN-2480 (37.5 mg/kg) 在肿瘤异种移植模型中是可以耐受的。 SK-MEL-30 异种移植模型受益于 MLN-2480 (12.5 mg) 和 TAK-733 (1 mg/kg) 的组合,但这两种药物本身都没有太大影响[2]。
剂量扩展阶段初步显示了Tovorafenib(MLN2480;BIIB-024;BSK1369;DAY-101;TAK-580;AMG-2112819)的疗效。在接受Q2D(每两日一次)RP2D(推荐二期剂量)治疗的BRAF突变阳性、未接受过RAF和MEK抑制剂治疗的16例患者中,8例(50%)观察到部分缓解。这种单药活性水平与类似情况下第一代药物的1期研究结果一致。 药代动力学(PK)分析表明,Tovorafenib(MLN2480;BIIB-024;BSK1369;DAY-101;TAK-580;AMG-2112819)具有中等快速吸收率,给药后总体中位Tmax为2-4小时。Q2D给药21天后的总体平均蓄积倍数为2.5倍。相比之下,在400 mg至800 mg剂量范围内,QW(每周一次)给药方案在体循环中基本未观察到明显蓄积。在测试的Q2D和QW剂量范围内,稳态AUC呈现近似剂量比例的增加。Tovorafenib的血浆终末半衰期(t1/2)约为70小时。[6] 与单药MLN2480相比,Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819)与 TAK-733 联用可抑制更广谱的RAS突变肿瘤模型生长(包括黑色素瘤和结直肠癌的原代人源肿瘤异种移植模型)。体外细胞增殖实验表明,该联合用药具有协同效应。蛋白质印迹分析证实,MLN2480能逆转TAK-733引起的MEK反馈激活,从而更协同地抑制MAPK通路。[2] |
| 酶活实验 |
激酶抑制试验[7]
抑制实验采用改良的hrf KinEASE酪氨酸激酶测定试剂盒进行。而不是提供试剂盒底物,我们纯化MEK135-393和生物素化它(MEK-B)内部使用birA酶。使用HP300e分配器将抑制剂分配到黑色384孔板中,并归一化至每孔1%的DMSO最终浓度。试剂盒测定缓冲液中加入MEK1SASA:BRAFKD:14-3-3和MEK1SASA:CRAFSSDD:14-3-3的终浓度为1 nM的纯化RAF, MEK1SASA: CRAFKD:14-3-3的纯化RAF为4 nM, MEK1SASA:ARAFSSDD-14-3-3的纯化RAF为10 nM,纯化生物素化MEK-B的终浓度为250 nM。使用Multidrop combi分配器将补充的激酶缓冲液分配到384孔板中,并在室温下与抑制剂孵育40分钟,然后使用Multidrop combi分配器分配200 uM ATP引发反应。用添加XL665和PAb Anti-phospho MEK1/2-Eu的试剂盒检测缓冲液在室温下淬火30min。使用PHERAstar微孔板读取器在665和620 nm处测量FRET信号比,并使用GraphPad Prism进行处理,该Prism适合Hill Slope为- 1的三参数剂量响应模型和Hill Slope适合数据的四参数剂量响应模型。测定一式三份,独立进行三次。 MLN2480(也称为 BIIB-024、TAK-580 和 AMG 2112819)是一种具有口服生物活性、有效且选择性的泛 Raf 激酶抑制剂,目前正在进行临床研究。在体内耐受的浓度下,MLN2480 会抑制某些 RAS 突变体和 BRAF 突变体临床前癌症模型中的 MAPK 通路信号传导。 |
| 细胞实验 |
在体外,MLN-2480 对野生型和 B-raf Val600Glu 均有效。在非常低的浓度下,MLN-2480 可激活磷酸化 MEK,但在较高浓度下,它会抑制相同的活性。高浓度的 MLN-2480 阻断人类恶性黑色素瘤 A-375 突变 B-raf Val600Glu 细胞系中的信号传导通路。研究发现 MLN-2480 的抑制作用因模型和遗传背景而异;它仅轻度抑制 PRAK。当 MLN-2480 和 TAK-733 在 NRAS 突变人类恶性黑色素瘤细胞系 (SK-MEL-2) 中组合时,观察到高水平的凋亡生物标志物。
采用基于ATP的细胞活力检测法,研究Tovorafenib(MLN2480;BIIB-024;BSK1369;DAY-101;TAK-580;AMG-2112819)与TAK-733联用对一系列BRAF和RAS突变黑色素瘤及结直肠癌细胞系的协同效应。通过蛋白质印迹分析比较了不同敏感程度的细胞系中MAPK通路信号及反应标志物的变化。[2] |
| 动物实验 |
C57BL/6J mice
12.5 mg/kg oral gavage Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819) was administered orally (tablet formulation), with patients fasting (except for water) for at least 2 h before and at least 2 h after taking their dose. Treatment was to be continued until disease progression, unacceptable toxicity, or the patient discontinued for any other reason, for a maximum duration of 12 months. Treatment could be continued beyond 12 months if it was determined that a patient would derive benefit from such continued therapy. In the dose escalation phase, a 3 + 3 design was used to evaluate Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819) administered with continuous dosing on Q2D and QW dosing regimens. Prior to the initiation of QW dose escalation, the initial cycle length of 22 days was changed to 28 days by protocol amendment to improve clinical feasibility and better facilitate future combination studies. Patients enrolled prior to this protocol amendment in an ongoing Q2D dose escalation cohort continued on the 22-day cycle schedule until the cohort was full and all patients had been evaluated for dose-limiting toxicity (DLT). For both Q2D and QW regimens, dose escalation progressed according to the incidence of DLT in the first treatment cycle (either 22 days or 28 days). DLTs were defined as: grade 4 neutropenia lasting ≥ 7 consecutive days; febrile neutropenia (defined as an ANC ≤ 1000 cells/μL and fever ≥ 38.5 °C) or documented infection ≥ grade 3 with ANC ≤ 1000 cells/μL; grade 4 thrombocytopenia (platelet count < 25,000/μL), tovorafenib-related thrombocytopenia requiring platelet transfusion, or tovorafenib-related bleeding requiring medical attention; treatment delays of ≥ 14 days due to any toxicity; ALT and AST toxicities (ALT or AST > 7.5 × ULN for greater than 14 days or ALT or AST > 7.5 × ULN accompanied by an elevation in total bilirubin of > 3 × ULN [not explained by obstruction] regardless of duration); nonhematological toxicity ≥ grade 3 (with the exception of: nausea, vomiting, and diarrhea except if they persisted at ≥ grade 3 for > 3 days despite adequate supportive care measures [at the investigator’s discretion, patients who experienced nausea, vomiting, or diarrhea after taking tovorafenib could receive antiemetic or antidiarrheal medication prior to subsequent doses]; isolated laboratory abnormalities ≥ grade 3 that resolved to ≤ grade 1 in ≤ 7 days without clinical sequelae or the need for therapeutic intervention; fatigue ≥ grade 3 for ≤ 7 days; development of keratoacanthomas or skin carcinoma unless unusually aggressive or metastatic), provided the site investigator considered such events were at least possibly related to study treatment. The MTD was defined as the highest dose level that generated DLT in 0/3 or 1/6 patients. On a case-by-case basis, the sponsor in collaboration with the principal investigators determined if intrapatient dose escalation was appropriate. Patients who had any dose reductions were not permitted to dose escalate. The starting dose for the Q2D dose escalation phase was 20 mg, which was equivalent to one-tenth of the highest non-severely toxic dose (HNSTD) established in monkey toxicology studies. Dose escalation included planned dose levels of 40 mg, 80 mg, 135 mg, 200 mg, and 280 mg. Once the MTD and/or RP2D of Q2D tovorafenib was established, patients with melanoma were enrolled into 1 of 6 Q2D melanoma expansion cohorts (approximately 16 patients per cohort), based on tumor genotype and treatment history (Supplementary Table S1). In addition, a seventh Q2D cohort was to enroll sufficient patients (approximately 16) with any advanced solid tumor (excluding lymphoma) to ensure that 12 patients completed protocol-specified dosing and PK assessments scheduled during cycle 1. The study was initially designed to investigate a Q2D schedule. Subsequently, a protocol amendment introduced planned QW dose escalation cohorts. The alteration in the dosing regimen from Q2D to QW was expected to reduce drug accumulation and increase Cmax while maintaining similar steady-state AUC. In addition, it was hypothesized that the increased Cmax might lead to a higher degree of pathway inhibition for a window of time within the dosing interval, without compromising overall dose density. Planned QW doses to be administered on days 1, 8, 15, and 22 of a 28-day cycle were a starting dose of 400 mg, followed by dose level increases of 200 mg (i.e., doses of 600 mg, 800 mg, and 1000 mg) in each subsequent cohort until the MTD/RP2D was reached. Once the MTD and/or RP2D of Q2D Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819) was established, and following a further protocol amendment, a single expansion cohort of up to 16 patients with NRAS-mutated cutaneous melanoma, naïve to prior therapy with RAF and MEK inhibitors was enrolled. [6] Safety, pharmacokinetic and pharmacodynamic assessments[6] Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 19.0 and were graded according to the National Cancer Institute (NCI) Common Terminology Criteria (CTC) for adverse events (CTCAE) (Version 4.03). The assessment period for treatment emergent adverse events (TEAEs) was from the first dose of study treatment to 30 days after the last dose of study medication, or until the start of subsequent antineoplastic therapy, whichever occurred first. Following baseline evaluation, response was assessed by investigators every two cycles by computed tomography or magnetic resonance imaging according to Response Evaluation Criteria in Solid Tumors (version 1.1). Serial blood samples were collected before and after Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819) dosing on days 1 and 21 (Q2D dosing) or days 1 and 22 (QW dosing) of cycle 1 for plasma PK analysis. In addition, for patients on Q2D schedules, predose or trough samples were collected on days 9 and 15 (Q2D dosing) or days 8 and 15 (QW dosing) to evaluate time to steady state. A validated liquid chromatography coupled to tandem mass spectroscopy (LC–MS/MS) method was used to quantify plasma concentrations of tovorafenib [24]. The concentrations of tovorafenib were determined using a fully validated bioanalytical method (QPS 96-1116) with a lower limit of quantification at 0.5 ng/mL in plasma. This bioanalytical method used protein precipitation extraction of tovorafenib and its stable labeled internal standard from human plasma with positive ionization mode in mass spectrometry. Plasma concentration–time analysis was performed using noncompartmental analysis. The plasma PK parameters were estimated using a validated version of Phoenix WinNonlin software (Version 6.3 or above, Pharsight Corporation, Raleigh, NC). Terminal half-life was calculated based on the equation: t1/2 = ln2/kel (kel = elimination rate constant determined by linear regression analysis of selected time points in the apparent terminal phase of the log plasma concentration versus time curve). Based on tissue availability, pharmacodynamic assays included assessment of pERK expression levels in paired biopsy samples (baseline and day 21) from patients in the melanoma dose expansion cohorts. The level of staining was assessed both by a pathologist (semi-quantitative measurements according to H-score assessment) and by quantitated image analysis. |
| 药代性质 (ADME/PK) |
Absorption
Tovorafenib steady-state maximum concentration (Cmax) is 6.9 µg/mL (23%) and the area under the concentration-time curve (AUC) is 508 µg*h/mL (31%). The time to reach a steady state of tovorafenib is 12 days (33%). Tovorafenib exposure increases in a dose-proportional manner. Tovorafenib median (minimum, maximum) time to achieve peak plasma concentration (Tmax) is 3 hours (1.5, 4 hours), following a single dose with tablets or oral suspension. No clinically significant differences in tovorafenib Cmax and AUC were observed following administration of tablets with a high-fat meal (approximately 859 total calories, 54% fat) compared to fasted conditions, but the Tmax was delayed to 6.5 hours. Route of Elimination Following a single oral dose of radiolabeled tovorafenib, 65% of the total radiolabeled dose was recovered in the feces (8.6% unchanged) and 27% of the dose was recovered in the urine (0.2% unchanged). Volume of Distribution Tovorafenib apparent volume of distribution is 60 L/m2 (23%). It crosses the blood-brain barrier. Clearance The apparent clearance is 0.7 L/h/m2 (31%). Metabolism / Metabolites Tovorafenib is primarily metabolized by aldehyde oxidase and CYP2C8 in vitro. CYP3A, CYP2C9, and CYP2C19 metabolize tovorafenib to a minor extent. Biological Half-Life Tovorafenib terminal half-life is approximately 56 hours (33%). Pharmacokinetics [6] Mean (± standard deviation) plasma concentration–time profiles of Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819) by QW dose group on days 1 and 22 of cycle 1 are shown in Fig. 2; cycle 1 day 22 plasma PK parameters are summarized by dose group in Table 4. Following multiple oral dosing of 600 mg QW, peak concentrations of tovorafenib were achieved at a median Tmax of 3 h post-dose (range 1–24 h) on cycle 1 day 22. Minimal to no apparent accumulation in terms of day 22 AUC168 over day 1 AUC168 was observed following repeated QW dosing. The mean plasma terminal half-life (t1/2) of tovorafenib was approximately 70 h (range 31–119 h) as defined in 20 evaluable patients receiving 600 mg QW. The relationship between dose and cycle 1 day 22 tovorafenib exposures (AUC168) is shown in Supplementary Figure S1. Steady-state exposures increased in an approximately dose-proportional manner over the 400 mg to 800 mg QW dose range with the 95% CI of the power model containing 1 (95% CI 0.55–2.04), with the coefficient of 1.30. For QW dosing regimens, minimum drug accumulation was observed and the geometric mean Rauc (accumulation ratio based on AUC0-last) was in the range of 1.03–1.09. With the Q2D dosing regimen at 200 mg, the geometric mean value of Rauc was ~ 2.55. Similar PK analyses were carried out by Q2D dose group (Supplementary Figs. S1 and S2, and Supplementary Table S10). Steady-state Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819)b AUC48 increased in an approximately dose-proportional manner over the dose ranges of 20 mg to 280 mg Q2D. While no apparent accumulation was observed with the QW dose regimens, Q2D administration resulted in approximately 2.5-fold accumulation in AUC48 at steady state. |
| 毒性/毒理 (Toxicokinetics/TK) |
The incidence of TEAEs and SAEs is summarized in Supplementary Table S5 and the most common TEAEs are listed in Table 2. Of note, only 1 of 149 treated patients (< 1%; Q2D dose expansion cohort) had squamous cell carcinoma of skin reported as a TEAE. The incidence of drug-related TEAEs according to dosing regimen is summarized in Supplementary Table S6. The two most common in the dose expansion phase were maculo-papular rash in the Q2D cohort (36%) and fatigue (42%) in the QW cohort. In the dose expansion phase, 68% of patients experienced a grade 3 or higher TEAE, including 73% of patients in the Q2D cohorts and 47% in the QW cohort. Grade 3 or higher TEAEs occurring in ≥ 5% of patients are listed in Supplementary Table S7. The two most commonly occurring overall were anemia (14%) and maculo-papular rash (8%). [6]
In the Q2D expansion cohorts, drug-related TEAEs of grade 3 or higher occurred in 33 of 80 patients (41%); the most common were maculo-papular rash (9%) and anemia (8%). In the QW expansion cohort, drug-related TEAEs of grade 3 or higher occurred in 4 of 20 patients (20%); the most common was hyperbilirubinemia (10%). In the dose escalation phase, drug-related treatment-emergent SAEs were reported in 2 of 30 patients (7%) in the Q2D cohort (280 mg dose level; grade 3 anemia in 1 patient, and grade 4 dyspnea and grade 5 respiratory failure in another patient) and 2 of 20 patients (10%) in the QW cohort (800 mg dose level; grade 3 rash, 1 patient, and grade 3 hyperbilirubinemia, 1 patient). In the dose expansion phase, drug-related treatment-emergent SAEs were reported in 12 of 80 patients (15%) in the Q2D cohorts and included acute kidney injury, macular rash, rash maculo-papular (grade 3 events in 2 patients each). In the QW dose expansion cohort, 4 of 19 patients (21%) had drug-related treatment-emergent SAEs, including grade 2 anemia and dyspnea in 1 patient, grade 2 nausea and grade 3 maculo-papular rash in another, and grade 3 erythema multiforme and macular rash in 1 patient each. In the dose expansion phase, 15 of 80 patients (19%) in the Q2D cohort had TEAEs resulting in permanent discontinuation of Tovorafenib (MLN2480; BIIB-024; BSK1369; DAY-101; TAK-580; AMG-2112819). These included maculo-papular rash and sepsis (2 patients [3%] each). In the QW cohort of the dose expansion phase, 4 of 19 patients (21%) had TEAEs resulting in permanent discontinuation, including atrial flutter, dyspnea, erythema multiforme, and fatigue (1 patient each). In the dose expansion phase, 19 of 99 patients (19%) had TEAEs leading to dose reduction including 17 of 80 patients (21%) in the Q2D cohorts and 2 of 19 (11%) in the QW cohort, the most common of which were maculo-papular rash (5 of 99 patients, 5%) and generalized rash (3 patients, 3%). There were 13 on-study deaths. The fatal SAEs associated with these deaths predominantly related to the underlying disease or complications thereof and are listed in Supplementary Table S8. Only one death, associated with respiratory failure in a patient in the 280 mg Q2D dose escalation cohort, was deemed by the study investigators to be treatment related.[6] Protein Binding Tovorafenib is 97.5% bound to human plasma proteins in vitro. |
| 参考文献 | |
| 其他信息 |
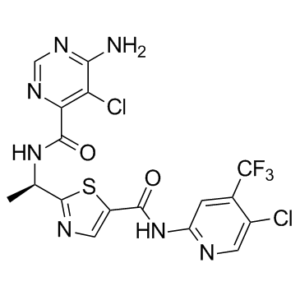
TAK-580 is a 1,3-thiazolecarboxamide that is 2-[(1R)-1-aminoethyl]-1,3-thiazole-5-carboxylic acid in which the carboxy group undergoes formal condensation with the amino group of 5-chloro-4-(trifluoromethyl)pyridin-2-amine and in which the amino group undergoes formal condensation with the carboxy group of 6-amino-5-chloropyrimidine-4-carboxylic acid. It is a pan-RAF kinase inhibitor which is currently in clinical development for the treatment of radiographically recurrent or progressive low-grade glioma in children and young adults. It has a role as an antineoplastic agent, an apoptosis inducer and a B-Raf inhibitor. It is a chloropyridine, an organofluorine compound, a secondary carboxamide, an aminopyrimidine, a pyrimidinecarboxamide and a 1,3-thiazolecarboxamide.
Tovorafenib (TAK-580) is under investigation in clinical trial NCT02723006 (Study to Evaluate the Safety, Tolerability, and Pharmacodynamics of Investigational Treatments in Combination With Standard of Care Immune Checkpoint Inhibitors in Participants With Advanced Melanoma). Tovorafenib is an orally available inhibitor of wild-type and certain mutant forms of A-Raf, B-Raf and C-Raf protein kinases, with potential antineoplastic activity. Upon administration, tovorafenib inhibits Raf-mediated signal transduction pathways, which may lead to an inhibition of tumor cell growth. Raf protein kinases play a key role in the RAF/MEK/ERK signaling pathway, which is often deregulated in human cancers and plays a key role in tumor cell proliferation and survival. Background: RAS mutant melanoma and colorectal cancer represent areas of significant unmet medical need. MLN2480 is an investigational class II RAF kinase inhibitor and TAK-733 is an investigational allosteric MEK kinase inhibitor; each of which is the subject of a single agent phase I clinical trial. The present studies have characterized the combination activity of these agents in BRAF mutant and RAS mutant preclinical models of melanoma and colorectal cancer. Methods: Combination effects of MLN2480 and TAK-733 on cell viability were studied using an ATP-based cell viability assay across a panel of BRAF and RAS mutant melanoma and CRC cell lines. Western blot analysis was used to compare effects on MAPK pathway signaling and response markers in cell lines showing a range of sensitivity to this combination. Pharmacodynamic responses and growth inhibitory effects of the combination were studied in xenografts of the same cell lines, as well as in primary human tumor xenografts, of RAS mutant melanoma and CRC. Results: MLN2480 inhibits MAPK pathway signaling in BRAF mutant and some RAS mutant preclinical cancer models at concentrations that are tolerated in vivo. MLN2480 is most potent in BRAF mutant melanoma models but also has single agent activity in some RAS mutant models. The combination of MLN2480 with TAK-733 inhibits the growth of a broader range of RAS mutant tumor models than single agent MLN2480, including primary human tumor xenograft models of melanoma and CRC. In vitro analysis of this drug combination in cell proliferation assays demonstrates synergistic activity. Western blot analysis demonstrated the effect of MLN2480 in reversing feedback activation of MEK in response to TAK-733, leading to more concerted MAPK pathway inhibition. Conclusions: The activity of the RAF kinase inhibitor MLN2480 in preclinical models of BRAF and RAS mutant melanoma and CRC provides a rationale for clinical testing. The combination of MLN2480 with the MEK inhibitor TAK-733 represents an additional strategy for clinical research within these tumor types.[2] Upon activation by RAS, RAF family kinases initiate signaling through the MAP kinase cascade to control cell growth, proliferation, and differentiation. Among RAF isoforms (ARAF, BRAF, and CRAF), oncogenic mutations are by far most frequent in BRAF. The BRAFV600E mutation drives more than half of all malignant melanoma and is also found in many other cancers. Selective inhibitors of BRAFV600E (vemurafenib, dabrafenib, encorafenib) are used clinically for these indications, but they are not effective inhibitors in the context of oncogenic RAS, which drives dimerization and activation of RAF, nor for malignancies driven by aberrantly dimerized truncation/fusion variants of BRAF. By contrast, a number of “type II” RAF inhibitors have been developed as potent inhibitors of RAF dimers. Here, we compare potency of type II inhibitors tovorafenib (TAK-580) and naporafenib (LHX254) in biochemical assays against the three RAF isoforms and describe crystal structures of both compounds in complex with BRAF. We find that tovorafenib and naporafenib are most potent against CRAF but markedly less potent against ARAF. Crystal structures of both compounds with BRAFV600E or WT BRAF reveal the details of their molecular interactions, including the expected type II–binding mode, with full occupancy of both subunits of the BRAF dimer. Our findings have important clinical ramifications. Type II RAF inhibitors are generally regarded as pan-RAF inhibitors, but our studies of these two agents, together with recent work with type II inhibitors belvarafenib and naporafenib, indicate that relative sparing of ARAF may be a property of multiple drugs of this class. [6] Upon activation by RAS, RAF family kinases initiate signaling through the MAP kinase cascade to control cell growth, proliferation, and differentiation. Among RAF isoforms (ARAF, BRAF, and CRAF), oncogenic mutations are by far most frequent in BRAF. The BRAFV600E mutation drives more than half of all malignant melanoma and is also found in many other cancers. Selective inhibitors of BRAFV600E (vemurafenib, dabrafenib, encorafenib) are used clinically for these indications, but they are not effective inhibitors in the context of oncogenic RAS, which drives dimerization and activation of RAF, nor for malignancies driven by aberrantly dimerized truncation/fusion variants of BRAF. By contrast, a number of "type II" RAF inhibitors have been developed as potent inhibitors of RAF dimers. Here, we compare potency of type II inhibitors tovorafenib (TAK-580) and naporafenib (LHX254) in biochemical assays against the three RAF isoforms and describe crystal structures of both compounds in complex with BRAF. We find that tovorafenib and naporafenib are most potent against CRAF but markedly less potent against ARAF. Crystal structures of both compounds with BRAFV600E or WT BRAF reveal the details of their molecular interactions, including the expected type II-binding mode, with full occupancy of both subunits of the BRAF dimer. Our findings have important clinical ramifications. Type II RAF inhibitors are generally regarded as pan-RAF inhibitors, but our studies of these two agents, together with recent work with type II inhibitors belvarafenib and naporafenib, indicate that relative sparing of ARAF may be a property of multiple drugs of this class. [7] Mechanism of Action Pediatric low-grade glioma, the most common childhood central nervous system (CNS) tumour, is often associated with BRAF genomic alterations, such as BRAF fusion or rearrangement. The BRAF kinase family is activated by RAS to phosphorylate MEK1/2, which phosphorylates ERK1/2 and promotes downstream signalling cascades that regulate multiple cellular processes, such as cell growth, proliferation, and differentiation. Oncogenic mutations in BRAF lead to an aberrant and hyperactivated RAS-RAF-MEK-ERK pathway, also known as the mitogen-activated protein kinase (MAPK) signalling pathway. Several RAF kinase inhibitors have been developed to treat cancers with BRAF mutations. These RAF inhibitors have been categorized into different "types" depending on their selectivity to a BRAF isoform and binding modes. Tovorafenib is a Type II RAF kinase inhibitor. RAF has a conserved three-residue segment (Asp-Phe-Gly) located at the N-terminus of the kinase activation loop called a DFG motif. In a state called a “DGF-out” conformation, the DFG motif is flipped in a way that reorients the phenylalanine residue, leaving a vacant site in which the drug can extend from the ATP site to insert a hydrophobic group. Tovorafenib is active against mutant BRAF V600E, wild-type BRAF, and wild-type CRAF kinases. Tovorafenib exhibited antitumor activity in cultured cells and xenograft tumour models harbouring BRAF V600E and V600D mutations, and in a xenograft model harbouring a BRAF fusion. Tovorafenib is not reported to induce paradoxical activation of the MAPK pathway. |
| 分子式 |
C17H12CL2F3N7O2S
|
|
|---|---|---|
| 分子量 |
506.29
|
|
| 精确质量 |
505.01
|
|
| 元素分析 |
C, 40.33; H, 2.39; Cl, 14.01; F, 11.26; N, 19.37; O, 6.32; S, 6.33
|
|
| CAS号 |
1096708-71-2
|
|
| 相关CAS号 |
|
|
| PubChem CID |
25161177
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.64
|
|
| LogP |
5.024
|
|
| tPSA |
164.02
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
32
|
|
| 分子复杂度/Complexity |
695
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
ClC1C(N([H])[H])=NC([H])=NC=1C(N([H])[C@]([H])(C([H])([H])[H])C1=NC([H])=C(C(N([H])C2C([H])=C(C(F)(F)F)C(=C([H])N=2)Cl)=O)S1)=O
|
|
| InChi Key |
VWMJHAFYPMOMGF-ZCFIWIBFSA-N
|
|
| InChi Code |
InChI=1S/C17H12Cl2F3N7O2S/c1-6(28-15(31)12-11(19)13(23)27-5-26-12)16-25-4-9(32-16)14(30)29-10-2-7(17(20,21)22)8(18)3-24-10/h2-6H,1H3,(H,28,31)(H2,23,26,27)(H,24,29,30)/t6-/m1/s1
|
|
| 化学名 |
2-[(1R)-1-[(6-amino-5-chloropyrimidine-4-carbonyl)amino]ethyl]-N-[5-chloro-4-(trifluoromethyl)pyridin-2-yl]-1,3-thiazole-5-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.67 mg/mL (1.32 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 6.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.67 mg/mL (1.32 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 6.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.67 mg/mL (1.32 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9752 mL | 9.8758 mL | 19.7515 mL | |
| 5 mM | 0.3950 mL | 1.9752 mL | 3.9503 mL | |
| 10 mM | 0.1975 mL | 0.9876 mL | 1.9752 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01425008 | Completed | Drug: MLN2480 | Melanoma Solid Tumor |
Millennium Pharmaceuticals, Inc. |
September 15, 2011 | Phase 1 |
| NCT02327169 | Completed | Drug: MLN2480 Drug: MLN0128 |
Advanced Nonhematologic Malignancies |
Millennium Pharmaceuticals, Inc. |
January 14, 2015 | Phase 1 |