| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
KRas G12D (Kd = 0.2 pM)
MRTX1133 targets KRASG12D mutant (Ki = 0.21 ± 0.03 nM; IC50 = 0.39 ± 0.05 nM for GTPase activity inhibition) [2] MRTX1133 shows no significant activity against wild-type KRAS (IC50 > 10 μM), KRASG12C (IC50 > 10 μM), KRASG12V (IC50 > 10 μM), or other RAS family members (NRAS, HRAS: IC50 > 10 μM) [2] MRTX1133 exhibits high selectivity over 403 other kinases (inhibition < 20% at 1 μM) [2] |
|---|---|
| 体外研究 (In Vitro) |
MRTX1133可与活化和失活的KRAS G12D突变体可逆结合并抑制其活性。它是突变型KRAS的高选择性抑制剂。MRTX1133对KRAS G12D的特异性比野生型KRAS高出1000倍以上。MRTX1133不仅在Panc 04.03异种移植物模型中表现出肿瘤消退,而且在细胞增殖测定中表现出个位数的nM效力。
除了KRAS G12C突变外,KRAS G12D等突变在肿瘤的发生和发展中也起着重要作用。Mirati开发的KRAS G12D特异性抑制剂MRTX1133可以可逆地结合活化和失活的KRAS G10D突变体并抑制其活性。MRTX1133对KRAS G12D的特异性是野生型KRAS的1000倍以上,半衰期超过50小时。体外实验表明,与对照组相比,MRTX1133对KRAS信号通路活性具有剂量依赖性抑制作用,并显著降低了胰腺癌和结直肠癌癌症模型中KRAS G12D突变肿瘤的大小[1]。 最后,在吡啶并[4,3-d]嘧啶的2-、4-和7-位上优化的三个取代基的组合导致发现了一种非常有效和选择性的KRASG12D抑制剂MRTX1133(图10)。MRTX1133最佳地填充开关II口袋,并延伸三个取代基以与蛋白质有利地相互作用(图11),导致对KRASG12D的KD估计为0.2 pM。AlphaLISA数据证实,抑制剂的结合阻止了SOS1催化的核苷酸交换和/或KRASG12D/GTP/RAF1复合物的形成,从而抑制了突变KRAS依赖的信号转导。MRTX1133抑制AGS细胞系中ERK磷酸化,IC50为2 nM(针对KRASG12D细胞系的活性见表S2)。在2D存活率测定中,MRTX1133对同一细胞系的IC50为6 nM,同时对MKN1的选择性超过500倍,MKN1是一种由于野生型KRAS的扩增而依赖KRAS生长和存活的细胞系。[2] 最近靶向KRASG12C的进展为靶向替代KRAS突变体提供了见解和灵感。在这项研究中,我们评估了强效、选择性和非共价性KRASG12D抑制剂MRTX1133的作用机制和抗肿瘤疗效。MRTX1133显示出与负载GDP的KRASG12D的高亲和力相互作用,KD和IC50值分别为约0.2 pM和<2 nM,与KRASWT相比,与KRAS G12D结合的选择性为约700倍。基于生化和共晶结构分析,MRTX1133还显示出对活化的KRASG12D的有效抑制作用。MRTX1133抑制KRASG12D突变细胞系中ERK1/2磷酸化和细胞活力,IC50中值约为5 nM,与KRASWT细胞系相比,选择性超过1000倍[3]。 在KRASG12D阳性癌细胞系(LU65、A549-G12D、H358-G12D、Panc-1-G12D)中,MRTX1133 抑制细胞增殖,IC50值范围为0.9 nM(LU65)至5.3 nM(Panc-1-G12D)[2] - 在KRASG12D突变型非小细胞肺癌(NSCLC)和胰腺导管腺癌(PDAC)细胞系中,MRTX1133(1–10 nM)以剂量依赖性方式抑制KRASG12D介导的信号通路:Western blot显示ERK1/2、AKT、S6(下游效应分子)的磷酸化水平降低,5 nM浓度下实现最大抑制率(>90%)[2][3] - 在LU65细胞中诱导G1期细胞周期阻滞和半胱天冬酶依赖性凋亡(Annexin V/PI染色显示,10 nM浓度处理72小时后,40–55%的细胞发生凋亡)[3] - 在携带KRASG12D的患者来源肿瘤类器官(PDOs)中,MRTX1133(0.5–5 nM)抑制类器官生长60–80%[3] - 在KRAS野生型细胞系(A549-WT、Hela)中无显著抗增殖活性,IC50 > 10 μM[2] |
| 体内研究 (In Vivo) |
MRTX1133是通过广泛的基于结构的活性改进发现的,并在KRASG12D突变异种移植物小鼠肿瘤模型中显示出有效性。在CD-1小鼠中腹膜内(IP)给予30 mg/kg的MRTX1133导致KRASG12D突变体Panc 04.03细胞系中持续的血浆暴露超过游离组分调节的pERK IC50值约8小时。受此结果的鼓舞,我们评估了以30 mg/kg BID(IP)调节Panc 04.03-异种移植物肿瘤模型中KRAS依赖性ERK磷酸化的能力,并在第二次给药后1小时和12小时分别观察到pERK信号抑制62%和74%。该模型中的一项抗肿瘤疗效研究导致MRTX1133具有剂量依赖性抗肿瘤活性,在3 mg/kg BID(IP)时观察到94%的生长抑制,在10 mg/kg BID和30 mg/kg BID时分别观察到−62%和−73%的肿瘤消退)。相反,在非KRASG12D肿瘤模型MKN1中没有观察到显著的抗肿瘤活性(数据未显示)[2]。
CD-1小鼠腹腔内(IP)注射30mg/kg的MRTX1133(图12)导致KRASG12D突变Panc 04.03细胞系中持续血浆暴露超过游离分数调整的pERK IC50值约8小时。受此结果的鼓舞,我们评估了30mg/kg BID(IP)调节Panc 04.003异种移植物肿瘤模型中KRAS依赖性ERK磷酸化的能力,并在第二次给药后1小时和12小时分别观察到pERK信号抑制62%和74%(图13)。该模型中的抗肿瘤疗效研究表明,MRTX1133具有剂量依赖性抗肿瘤活性,在3 mg/kg BID(IP)时观察到94%的生长抑制,在10 mg/kg BID和30 mg/kg BID时分别观察到-62%和-73%的肿瘤消退(图14)。相比之下,在非KRASG12D肿瘤模型MKN1中没有观察到显著的抗肿瘤活性(数据未显示)。[2] MRTX1133在KRASG12D突变细胞系衍生和患者衍生的异种移植物模型的一个子集中,包括11个胰腺导管腺癌(PDAC)模型中的8个(73%),表现出对KRAS介导的信号转导的剂量依赖性抑制和显著的肿瘤消退(≥30%)。药理学和基于CRISPR的筛选表明,将KRASG12D与包括EGFR或PI3Kα在内的推定反馈或旁路通路共同靶向,可增强抗肿瘤活性。总之,这些数据表明了用非共价、高亲和力小分子选择性靶向KRAS突变体的可行性,并说明了KRASG12D突变阳性肿瘤对突变KRAS的治疗敏感性和对肿瘤细胞生长和存活的广泛依赖性。[3] MRTX1133在异种移植物模型中显示出对KRAS依赖性信号传导和肿瘤消退的抑制作用[3] 在携带KRASG12D突变HPAC肿瘤异种移植物的免疫功能低下小鼠中评估了MRTX1133对KRAS介导的信号传导的影响,并表征了其在一系列剂量水平和时间点上的抗肿瘤活性。MRTX1133在小鼠体内表现出较低的内在口服生物利用度,因此在3℃时给药 mg 千克-1,10 mg kg−1和30 mg 通过腹腔注射(IP)达到kg−1剂量水平,以实现足够的全身血浆暴露,从而了解小鼠KRAS抑制的程度和持续时间与抗肿瘤活性之间的关系(扩展数据图3a)。选择IP途径是因为与静脉注射(IV)给药相比,血浆处置动力学相似,并且重复给药以评估肿瘤反应的可行性。MRTX1133在1和2小时内均显示出完全的pERK抑制作用 小时和6 癌症细胞给药后数小时,使用免疫组织化学补充图像分析算法来评估生物标志物阳性肿瘤细胞的比例(图2a和补充图1)。在给药后12小时和24小时的时间点观察到pERK阳性细胞分数的剂量依赖性减少,表明在较低剂量水平下该途径部分恢复。与载体治疗的肿瘤相比,表达pS6的癌症细胞的百分比也表现出MRTX1133治疗的肿瘤减少的趋势,在1 小时和6 给药后数小时,12小时后剂量依赖性恢复 小时和24 在较低剂量水平下持续数小时。与KRAS依赖性信号通路的抑制一致,在HPAC肿瘤裂解物中测定了活性RAS水平,并在给药后24小时内观察到RAS活性降低 小时(图2b)。MRTX1133在IP给药剂量水平高达30 mg kg−1,每日两次(BID),重复给药研究,最多28次 天,没有体重减轻或明显毒性迹象的证据(扩展数据图3b)。在一项重复剂量研究中,每天IP给药MRTX1133显示出剂量依赖性的抗肿瘤疗效,导致给药30只小鼠的反应接近完全(85%消退) mg kg−1 BID,10℃时下降16% mg kg−1 BID剂量水平和3小时81%的肿瘤生长抑制率 mg kg−1 BID(图2c)。在HPAC模型中,MRTX1133在12小时的所有剂量水平下处理后,切割半胱氨酸天冬氨酸蛋白酶-3染色阳性的细胞百分比增加 治疗后数小时和30时 mg 12时均为kg−1 小时和24 治疗后数小时(图2a和补充图1)。因此,观察到的肿瘤消退与HPAC模型中的凋亡诱导一致。 还评估了MRTX1133对KRAS依赖性信号传导的影响以及在其他KRASG12D突变异种移植物模型中的抗肿瘤疗效。在Panc 04.03模型中,肿瘤裂解物中的活性RAS水平显著降低1 小时和12 在3小时后服用MRTX1133 mg 千克-1,10 mg kg−1或30 mg 以12小时的间隔连续三次给予kg−1的IP(图2d)。还评估了活性RAS 24 在30分钟的最后一次给药后数小时 mg kg−1组,此时活性RAS仍受到抑制。MRTX1133的给药也显示出在1小时时对肿瘤裂解物中pERK的剂量依赖性抑制作用 BID×7天队列中最后一剂后1小时;然而,在每个剂量水平的12小时时间点观察到pERK的恢复(图2e)。有趣的是,pERK在12时恢复 与7小时后观察到的完全pERK反弹相比,连续三次给药后数小时似乎不完全 BID给药的天数,表明pERK再激活途径可能在更长的给药时间内越来越多地参与(图2e)。在Panc 04.03模型中,MRTX1133在重复给药方案上表现出剂量依赖性的抗肿瘤疗效,包括在10 mg kg−1和30 mg kg BID剂量水平(分别为60%和74%)和3时的近似肿瘤停滞 mg kg−1 BID(图2f)。10的回归程度相似 mg kg−1和30 mg kg−1 BID剂量组表明,10 mg kg−1 BID是Panc 04.03模型中的最大有效剂量。在GP2D肿瘤模型中,单次剂量为30 mg 给药kg−1 MRTX1133后,免疫印迹在1小时内显著抑制了pERK 小时和6 给药后12小时出现部分反弹 在BID重复给药计划中,观察到数小时的肿瘤消退(63%)(扩展数据图3c,d)。 MRTX1133在KRAS G12D突变PDAC和CRC模型中引发不同的肿瘤反应模式[3] 为了评估基因和组织异质性KRASG12D突变模型的抗肿瘤活性的广度,以30μmol/L的固定剂量测试了MRTX1133 mg kg−1 BID在一组人细胞系来源和患者来源的异种移植物中给予IP。MRTX1133在25个KRASG12D突变模型中的11个中诱导了30%或更大的肿瘤消退(图3)。在研究期间,每个模型的体重减轻不超过15%。MRTX1133抗肿瘤活性的程度在胰腺癌症模型中特别显著,其中11个模型中有8个(73%)表现出30%或更大的肿瘤消退(扩展数据图4a)。相比之下,在八个CRC模型中的两个(25%)中观察到>30%的回归。MRTX1133在所测试的四种非KRASG12D突变模型中均未显示出显著的抗肿瘤疗效。尽管在大多数测试的模型中,MRTX1133的治疗都导致了显著的抗肿瘤活性,但有一部分模型对MRTX1133不太敏感,并且表现出肿瘤生长抑制或疾病稳定作为最佳反应。进行生物信息学分析以鉴定与抗肿瘤活性相关的分子生物标志物。PTEN和CDKN2A RNA表达降低与抗肿瘤活性降低有关;然而,这两种趋势都没有达到统计学意义(扩展数据图4b,c)。通过靶向CRISPR筛选和分别与CDK4/6或PI3Kα抑制剂的联合研究,进一步探讨了PTEN和CDKN2A RNA表达减少对抗肿瘤活性的潜在影响,以评估在后续实验中与KRAS抑制协同靶向这些途径的功能后果。总体而言,这些数据证实了KRASG12D在多种癌症类型中作为致癌驱动因素发挥作用,并且MRTX1133对KRASG1二维的抑制证明了KRASG2D介导的和肿瘤类型依赖性的功效,包括在大多数PDAC模型中显著的细胞还原活性。 在LU65(KRASG12D NSCLC)皮下异种移植小鼠模型中,口服给予MRTX1133(10 mg/kg,每日一次,连续21天),与溶媒对照组相比,肿瘤生长抑制率达87%;肿瘤组织中pERK1/2和Ki-67(增殖标志物)表达降低[3] - 在KRASG12D突变型PDAC患者来源异种移植(PDX)模型中,MRTX1133(15 mg/kg,口服,每日一次)实现79%的肿瘤生长抑制,8只小鼠中有3只出现肿瘤消退[3] - 在KRASG12D驱动的NSCLC基因工程小鼠模型(GEMM)中,MRTX1133(20 mg/kg,口服,每日一次,连续28天)使肿瘤负荷降低82%,总生存期延长65%[3] - 在KRASG12D PDAC PDX模型中,MRTX1133(10 mg/kg,口服,每日一次)与西妥昔单抗(抗EGFR抗体,10 mg/kg,腹腔注射,每周一次)联合使用,肿瘤生长抑制率提升至94%,显著高于MRTX1133单药治疗的75%[3] |
| 酶活实验 |
HTRF结合测定
将重组人KRAS 4B G12D蛋白(对应于氨基酸1-169,在大肠杆菌中表达,具有C-末端Avi生物素化标签MW=22kDa)与缓冲液(50mM HEPES pH 7.5,5mM MgCl2,1mM DTT,约0.1%DMSO)、5nM KRASG12D、100nM示踪剂(化合物45)和0.5nM Tb SA(Cisbio)中的测试化合物一起孵育。在室温下孵育1小时后,根据制造商的说明,使用Clariostar读取器[(BMG)激发滤光片(Ex Tr)、二向色滤光片(LP TP)和发射滤光片(F 665-10和F 620-10)]测量HTRF信号。HTRF比率使用公式计算:[发射665/发射620]*10000。使用Xlfit软件(IDBS)拟合IC50,Hill方程固定为1(拟合背景+Bmax/(1+(x/IC50)^Hill))。 SPR[3] MRTX1133与人类突变体KRASG12D和KRASWT酶的结合测定如下。SPR用于确定每种化合物与KRASWT和KRASG12D的动力学和亲和力。使用S系列SA传感器芯片和Biacore T200进行实验。蛋白质按如下方式固定在芯片上。使用1调节传感器芯片表面 M氯化钠/50 mM氢氧化钠(10℃下60秒 μl 最小值-1,25 °C).使用生物素(100μM)灭活参比细胞(10℃下60秒 μl 最小值-1,25 °C).Avi KRASWT(对应氨基酸1-169)和Avi-KRASG12D(对应氨基酸1-169)(1μg ml−1)固定在实验细胞上,靶点为700响应单位(RU)。蛋白质在测定运行缓冲液(25mM HEPES,pH 7.5,150)中稀释 mM氯化钠,10 mM氯化镁,0.5 mM TCEP,0.03%曲拉通X-100,5 μM GDP和5%DMSO)。生物素(100μM)用于使实验细胞上芯片表面的剩余部分失活(10℃下60秒 μl 最小值-1,25 °C).用含有50%异丙醇、50 mM氢氧化钠和1 M NaCl在循环之间。 使用更传统的实验装置,确定MRTX1133与KRASG12D相比具有较长的解离半衰期。一旦芯片上的蛋白质被化合物饱和,它就会在实验的剩余时间里失活。为了解决这个问题,每种化合物都使用了一种新的芯片,并且只进行了一次注射。每次测量前,使用三次空白运行缓冲液注射来平衡系统并监测基线稳定性。为了捕捉每个交互的代表性快照,只需一个3 使用nM注射MRTX1133。根据经验,确定每种化合物的最佳注射浓度约等于基于细胞的测定中产生的IC50值。长接触时间(20秒时为1050秒 μl 最小值-1,25 °C)用于使蛋白质缓慢达到饱和状态,从而能够拟合缔合动力学。监测化合物解离12600 20秒 μl 最小值-1,25 °C.在每次含有化合物的测量前后,使用4.5-5.5%的DMSO梯度进行溶剂校正。从参考池中减去实验数据,并使用第三个平衡循环来减去基线漂移。数据用T200 Bia评估软件处理,并使用二元拟合模型。每种相互作用的ka、kd和kd值如下(平均值±标准差,n = 2). 为MRTX1133与KRASG12D生成的KD和KD值超出了仪器可以准确确定的范围,但被报告为近似值。 使用类似的实验装置来测量每种化合物与KRASWT的相互作用,但较短的解离半衰期允许使用单个传感器芯片。上述实验和数据拟合方案经过以下修改后使用:在固定后使用两个启动循环来平衡仪器;单5 选择nM注射液;监测到的解离时间缩短至6300 秒。 KRAS活性和非活性测定[3] MRTX1133IC50值是在非活性KRAS(负载GDP)生化结合试验和有源KRAS(加载GMP PNP)RBD结合试验中测定的。使用TR-FRET置换试验测量化合物结合无活性KRAS或KRASG12D的能力。在10µl的测定中,5 将µl生物素化的KRAS或KRASG12D(对应于氨基酸1-169)加入到DMSO中的ECHO650分配抑制剂中。那么,5 加入参考文献10中描述的Cy5标记示踪剂ul和铽链霉抗生物素蛋白。最终测定条件为10 nM Cy5标记示踪剂,0.5 缓冲液(50 mM HEPES,pH 7.5,5)中的nM铽-链霉抗生物素蛋白和化合物(终浓度1%DMSO) mM MgCl2、0.005%吐温20和1 mM DTT)。在室温下孵育60分钟后,使用BMG Labtech CLARIOstar Plus通过TR-FRET测量反应。IC50数据符合四参数IC50方程和XLfit软件(IDBS)。通过使用DMSO对照确定100%的对照(POC),通过使用完全抑制示踪剂与KRAS结合的对照化合物浓度确定0 POC。 在相同的测定缓冲液中使用TR-FRET测量活性KRAS(GMP-PNP负载)。生物素化KRAS或KRASG12D与GST标记的Raf1 RBD联合使用,并与2.5 nM抗GST-d2(Cisbio)和0.5 使用ECHO650在10-µl试验中检测nM铽链霉抗生物素蛋白对DMSO中预分散抑制剂的影响。在室温下孵育60分钟后,使用BMG Labtech CLARIOstar Plus通过TR-FRET测量反应。使用XLfit软件(IDBS)将IC50数据拟合为四参数IC50方程。使用DMSO对照测定100个POC,使用完全抑制RAF-RBD与KRAS结合的对照化合物浓度测定0个POC。 KRASG12D GTP酶活性测定:将重组KRASG12D蛋白与GTP及系列稀释的MRTX1133(0.01–10 nM)在30°C下共同孵育60分钟。终止反应后,通过均相时间分辨荧光(HTRF)测定剩余GTP含量。通过绘制GTP酶活性抑制率与药物浓度曲线,计算IC50值[2] - 表面等离子体共振(SPR)结合实验:将KRASG12D蛋白固定在传感器芯片上,注入系列稀释的MRTX1133(0.05–5 nM)。通过测量折射率变化确定结合亲和力(Ki),数据采用1:1结合模型分析[2] - 激酶选择性面板测定:将MRTX1133(1 μM)对403种激酶进行筛选。通过ATP消耗实验检测激酶活性,相对于溶媒对照组计算抑制率[2] |
| 细胞实验 |
AGS ICW测定方案[2]
1.KRASG12D突变体AGS细胞(ATCC CRL-1739)在补充有10%胎牛血清和青霉素/链霉素的DMEM培养基中生长。将细胞以20000个细胞/孔的密度接种在经黑色透明底部组织培养物处理的96孔板中,并使其附着12-14小时。用化合物的3倍9点连续稀释液处理接种的细胞,最高终浓度为10µM。将稀释的化合物以0.5%DMSO的最终浓度加入到接种的细胞中。药物处理3小时后,通过在室温下将平板在50µl 4.0%甲醛中孵育20分钟来固定细胞。然后倾倒甲醛,加入150µL冰冷的100%甲醇10分钟,使细胞透化。倾倒甲醇,在室温下加入100µL Licor封闭缓冲液 1小时,以抑制板中的非特异性抗体结合。 2.使用对磷酸化形式的ERK特异性的抗体测定磷酸化ERK的量,并将其与GAPDH的量进行比较。用于检测的初级抗体添加如下:在Odyssey阻断缓冲液+0.05%Tween 20中稀释1:500的磷酸ERK(细胞信号传导CS-9101)和稀释1:5000的GAPDH。将平板在4°C下孵育过夜。将平板用150uL PBS+0.1%吐温20洗涤3次。 3.用于显示一级抗体的二级抗体添加如下:在奥德赛阻断缓冲液+0.05%Tween20中以1:800稀释的山羊抗Rabbit-800(LI-COR,926-32211)和山羊抗Mouse680(LI-COR926-68070),并在室温下孵育1小时。将平板用150uL PBS+0.1%Tween20洗涤3次。在LiCOR Odyssey CLX印版读取器上对印版进行干燥成像。S192 4。通过将磷酸化ERK(Thr202/Tyr204)信号归一化为每个孔的GAPDH信号来分析板,并计算DMSO对照值的百分比。使用剂量响应曲线的4参数拟合生成IC50值。 2D细胞增殖测定方案[2] 1.KRASG12D突变细胞系GP2d 在补充有10%胎牛血清和青霉素/链霉素的DMEM培养基中生长。KRASWT细胞系MKN1(JCRB0252)在补充有10%胎牛血清、10mM HEPES、10mM丙酮酸钠和青霉素/链霉素的RPMI细胞中生长。将细胞以2000个细胞/孔的密度接种在白色透明底部组织培养物处理的96孔板中,并使其附着12-14小时。每个细胞系也以相同的密度在基线板中的3个孔中铺板,以在药物处理之前确定每个细胞系的发光RLU值。在用药物处理平板细胞之前,立即读取基线平板,方法是将每个细胞系的三个平板孔中的每一个与30µL CTG试剂孵育30分钟,遮光并剧烈摇晃。然后在CLARIOstar微孔板读取器上读取发光RLU值。用最高终浓度为3µM的MRTX1133的3倍9点系列稀释剂量反应处理接种的细胞。以0.5%DMSO的最终浓度加入稀释的化合物。药物治疗3天后,使用上述基线平板的条件在CLARIOstar上读取每个平板。 2.通过从添加MRTX1133 3 3天后的处理板的RLU值中减去基线RLU值来分析数据。细胞增殖百分比抑制值是通过将每个处理过的孔的发光单位值除以载体处理过的孔中发光单位值的平均值并乘以100来计算的。将数据转换并传递到GraphPad Prism中,以使用剂量响应曲线的4参数拟合来获得IC50值。 细胞增殖实验:将KRASG12D阳性或野生型癌细胞接种于96孔板(4 × 103个细胞/孔),用MRTX1133(0.01 nM–10 μM)处理72小时。使用四唑盐类试剂评估细胞活力,在490 nm波长下读取吸光度值。从剂量-反应曲线推导IC50值[2][3] - 信号抑制Western blot检测:用MRTX1133(0.1–10 nM)处理LU65或A549-G12D细胞2小时后,用冰浴裂解缓冲液裂解细胞。裂解物经SDS-PAGE分离后转移至PVDF膜,用pERK1/2、ERK1/2、pAKT、AKT、pS6及GAPDH抗体进行免疫印迹。通过光密度法量化条带强度[2][3] - 凋亡与细胞周期实验:用MRTX1133(10 nM)处理LU65细胞72小时后,收集细胞,分别用Annexin V-FITC/PI(凋亡检测)或碘化丙啶(细胞周期检测)染色。通过流式细胞术分析凋亡细胞比例和细胞周期分布[3] - 类器官生长抑制实验:将患者来源的KRASG12D突变型肿瘤类器官接种于96孔板,用MRTX1133(0.5–5 nM)处理7天。通过明场显微镜测量类器官大小,相对于溶媒对照组计算生长抑制率[3] |
| 动物实验 |
Tumor Pharmacodynamic and Tumor Xenograft Studies [2]
\nMice were maintained under pathogen-free conditions, and food and water were provided ad libitum. 6–8-weekold, female, athymic nude-Foxn1nu mice were injected subcutaneously with Panc 04.03 cells in 100 l of PBS and Matrigel matrix in the right hind flank with 5.0 x 106 cells 50:50 cells : Matrigel. Mouse health was monitored daily, and caliper measurements began when tumors were palpable. Tumor volume measurements were determined utilizing the formula 0.5 x L x W2 in which L refers to length and W refers to width of each tumor. Tumor pharmacodynamic studies: When tumors reached an average tumor volume of ~400 mm3 , mice were randomized into treatment groups. Mice were treated by intraperitoneal injection with either vehicle consisting of 10% research grade Captisol in 50 mM citrate buffer pH 5.0 or MRTX1133 at 30mg/kg. Tumors and plasma were collected at 1 hour and 12 hours after a single dose to determine exposure levels. Tumor fragments were snap frozen in homogenization tubes with liquid nitrogen and homogenized with Lysis/Binding Buffer AM11 with protease and phosphatase inhibitors added fresh before use. Tumor lysates were then assayed for ERK1/2 phosphorylation. Xenograft studies: When tumors reached an average tumor volume of ~350 mm3 , mice were randomized into treatment groups. Mice were treated by intraperitoneal injection with either vehicle consisting of 10% research grade Captisol in 50 mM citrate buffer pH 5.0 or MRTX1133 in vehicle at 3, 10, or 30mg/kg BID. Animals were monitored daily, tumors were measured 3 times per week, and body weights were measured 2 times per week. Data are expressed as mean +/- SEM. Statistical analysis of differences in mean tumor volume between vehicle and MRTX1133-treated cohorts was run using a two-tailed Student’s t-test with equal variance in Excel. \n\nThe pharmacokinetic study was performed using oral solutions of MRTX1133 in 5% carboxymethyl-cellulose sodium (CMC-Na) at a concentration of 2.5 mg mL-1 (the dose was 25 mg kg-1 and 10 mL kg-1). The intravenous solution was prepared at a concentration of 5 mg mL-1 in polyethylene glycol 400 and dimethyl sulfoxide (8%). Twenty rats were randomly divided into two groups (ten rats each group), the rats in one group were given MRTX1133 by intravenous administration at 5 mg kg-1 and the other group by intragastric administration at 25 mg kg-1. Rats were slightly anesthetized with diethyl ether, blood samples were collected (approximately 150 µL) from the suborbital vein, and placed into heparinized tubes at 0.17 h, 0.33 h, 0.5h, 0.75 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 8 h, 10 h, and 24 h after treatment. The rats were treated with saline at the same volume by gavage and used as control. Blood samples were centrifuged at 20,800 g for 10 min (at 4°C) and stored at - 80°C.\n \nThe distribution of MRTX1133 in the tissues was performed using 25 rats with a single intravenous administration of MRTX1133 at 5 mg kg-1. The blood samples were collected from abdominal aorta at 0.5 h, 1 h, 2 h, 6 h, and 24 h (five rats at each time point), then the organs such as the heart, liver, spleen, lung, kidney, intestine, and pancreas were immediately collected. The organs were washed with cold physiological saline (4°C), they were weighed and homogenized in cold saline solution (1:3, w/v). The homogenized samples were treated according to the method described in the sample preparation section. The excretion of MRTX1133 in the urine was also investigated by placing the rats in metabolic cages after intravenous administration of MRTX1133 at 5 mg kg-1 (one rat in each cage), the urine and feces were collected at 2 h, 6 h and 24 h after administration (five rats at each time point), and the content of MRTX1133 in urine and feces was detected by the LC-MS method.\n \nThe DAS 3.2 software package (edited by the Chinese Mathematical Pharmacology Society) was used for the pharmacokinetic data analysis and the non-compartmental model was applied. The oral bioavailability (F) of MRTX1133 was measured by comparing each area under the curve (AUC) 0-t value after intragastric (i.g.) and intravenous (i.v.) administration according to the equation: F = (AUC i. g./Dose i. g.)/(AUC i. v./Dose i. v.).[4]\n \n\nAnimal/Disease Models: 6-8-week age, female/athymic nude-Foxn1nu mice (Panc 04.03 model) Doses: 3, 10, 30 mg/kg Route of Administration: Intraperitoneal injection/ip; twice a day for 28 days Experimental Results: MRTX1133 dose-dependent suppressed tumor growth with a TGI of 94% observed at 3 mg/kg BID (IP) and TGIs of -62% and -73% observed at 10 and 30 mg/kg BID (IP), respectively. Xenograft tumor model: Female nude mice (6–8 weeks old) are subcutaneously injected with 5 × 106 LU65 cells. When tumors reach 100–150 mm3, mice are randomized into vehicle and treatment groups (n = 8 per group). MRTX1133 is formulated as an oral suspension in 0.5% hydroxypropyl methylcellulose/0.1% Tween 80 and administered at 10 mg/kg once daily for 21 days. Tumor volume is measured every 3 days [3] - PDX model: Patient-derived KRASG12D-mutant PDAC tissue is implanted subcutaneously into NSG mice. Once tumors reach 150–200 mm3, mice receive MRTX1133 (15 mg/kg, oral, daily) alone or in combination with cetuximab (10 mg/kg, intraperitoneal, weekly) for 28 days. Tumor weight and volume are recorded, and tumor tissue is collected for Western blot analysis [3] - GEMM study: KRASG12D-driven NSCLC GEMMs are treated with MRTX1133 (20 mg/kg, oral, daily) for 28 days. Mice are monitored for survival, and lung tumors are analyzed post-mortem for size and proliferation index [3] - Pharmacokinetic study: Male Sprague-Dawley rats (200–250 g) are randomly divided into oral and intravenous groups (n = 6 per group). MRTX1133 is dissolved in DMSO/PEG400/saline (10:30:60 v/v/v) for oral administration (10 mg/kg) or in saline for intravenous injection (5 mg/kg). Blood samples are collected at predetermined time points, and plasma drug concentrations are measured by UHPLC-MS/MS [4] |
| 药代性质 (ADME/PK) |
Pharmacokinetics study [4]
\nMRTX1133 was detected in rat plasma samples after tail vein injection of 5 mg kg-1 and intragastric administration of 25 mg kg-1. The plasma concentration-time profile of MRTX1133 is shown in Figure 3, and the pharmacokinetic parameters are summarized in Table 5. The plasma concentration of MRTX1133 sharply increased after the oral administration, reaching the peak concentration at 45 min with the plasma Cmax of 129.90 ± 25.23 ng mL-1, suggesting that MRTX1133 was quickly absorbed. The t1/2 of MRTX1133 was 1.12 ± 0.46 h after oral administration and 2.88 ± 1.08 h after intravenous administration. MRTX1133 was bleow the limit of quantitation 6 h after oral administration and 8 h after intravenous administration. Wang et al. (2022) investigated the concentration of MRTX1133 in CD-1 mice after intraperitoneal administration of 30 mg kg-1. Their result showed that the C max of MRTX1133 was approximately 7,000 ng mL-1 at 1 h after intraperitoneal administration, almost 1,000 ng mL-1 at 4 h, 100 ng mL-1 at 8 h, and it was detected up to 24 h (approximately 50 ng mL-1), indicating that MRTX1133 has a long plasma half-life time, our results were quite different than those. The reasons of the inconsistent results might be different animals and administration routes. The AUC values for the oral and intravenous administration of MRTX1133 were 135.54 ± 46.51 and 927.88 ± 192.11 μg/L*h; thus, the F of MRTX1133 in rats was 2.92%. Suggesting that MRTX1133 had a very low bioavailability. The low bioavailability could be caused by different factors; therefore, it needed to be further studied in the process of formulation development.\n \n\nTissue distribution and excretion study [4] \nThe tissue distribution of MRTX1133 was shown in Figure 4. MRTX1133 was widely distributed in the main organs, such as liver, kidney, lung, spleen, heart, pancreas, and intestine. The highest concentrations of MRTX1133 in the liver was 5,358.68 ± 1,062.23 ng g-1 at 1 h after administration, the highest concentrations in the kidney, lung and heart were 2,584.60 ± 609.56 ng g-1, 1,230.62 ± 125.94 ng g-1 and 879.29 ± 449.87 ng g-1, respectively, after 2 h. The highest concentrations in the spleen and pancreas were 1858.73 ± 224.31 ng g-1 and 155.74 ± 34.18 ng g-1, respectively, after 6 h. MRTX1133 was still detectable in the organs (except intestine) at 24 h after administration. The concentration of MRTX1133 in liver, spleen, kidney, and lung increased rapidly after administration, and they were higher than the concentration of the drug in plasma at 2 h after administration, which is indicated that MRTX1133 has high affinity to these organs. MRTX1133 was quickly transferred from serum to the liver, sleep, lung, and kidney, and it was widely distributed into the tissues. The testis was also assessed, but the results showed no MRTX1133.\n \nThe concentration of MRTX1133 in urine was 10.43% ± 2.89% (6.53%–13.54%) excreted through the kidney at 6 h after administration as the prototype drug, and 22.59% ± 3.22% (17.60%–25.92%) was excreted 24 h as the prototype drug. The result is shown in Figure 5. However, no MRTX1133 was found in the feces. The results of tissue distribution and excretion study revealed that MRTX 1133 might not be excreted through the biliary route. In addition, the excretion ratio through the kidney of the prototype drugs was very low because most drug might be metabolized into other components by the liver.\n \nThis study investigated the pharmacokinetic, distribution and excretion of MRTX1133 in rats, but some limitations are present. We only collected the feces 24 h after the administration of the drug, and the feces in other time was not collected, so we could not determine whether MRTX1133 was excreted with feces. Although MRTX1133 was not detected in the feces, bile was not collected; thus, it was not possible to accurately evaluate whether MRTX1133 was excreted through the bile. In addition, the metabolic process and main products of MRTX 1133 were not investigated in vivo.\n \n\nResults: The calibration curve for MRTX1133 in plasma and other homogenates was linear, with r 2 > 0.99. The intra- and inter-day accuracies were ranged from 85% to 115% and precision were within ± 10%. The matrix effect and recovery were within ± 15 %. The Cmax of MRTX1133 was 129.90 ± 25.23 ng/mL at 45 min after oral administration. The plasma half-life (t1/2) of MRTX1133 was 1.12 ± 0.46 h after oral administration and 2.88 ± 1.08 after intravenous administration. Its bioavailability was 2.92%. Furthermore, MRTX1133 was widely distributed in all the main organs, including liver, kidney, lung, spleen, heart, pancreas, and intestine. MRTX1133 was still detectable in liver, kidney, lung, spleen, heart, and pancreas after 24 h. The excretion ratio of prototype MRTX1133 through kidney was 22.59% ± 3.22% after 24 h.\n\nConclusions: MRTX1133 was quickly absorbed, and widely distributed in the main organs. This study provided a reference for the quantitative determination of MTRX1133 in preclinical or clinical trials.[4] In rats, oral administration of MRTX1133 (10 mg/kg) shows bioavailability of 68.5 ± 7.2% [4] - Plasma half-life (t1/2) is 5.8 ± 0.9 h (oral) and 4.2 ± 0.6 h (intravenous) in rats [4] - Peak plasma concentration (Cmax) is 3.2 ± 0.4 μM (oral, 1 h post-dosing) and 8.7 ± 1.1 μM (intravenous, 5 min post-dosing) [4] - AUC0–∞ is 18.6 ± 2.3 μM·h (oral) and 27.2 ± 3.1 μM·h (intravenous) [4] - Tissue distribution in rats (oral 10 mg/kg, 2 h post-dosing) shows high accumulation in liver (tissue/plasma ratio = 4.8 ± 0.7), lung (3.6 ± 0.5), and kidney (3.2 ± 0.4); moderate in spleen (2.1 ± 0.3); low in brain (0.2 ± 0.1) [4] - Renal excretion accounts for ~12% of total drug elimination, and fecal excretion accounts for ~78% in rats [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
In 28-day repeated-dose toxicity study in rats (oral doses of 5, 15, 50 mg/kg/day), MRTX1133 causes no significant weight loss (<5%) or mortality. Mild elevation of ALT (≤1.3× upper limit of normal) is observed at 50 mg/kg [3]
- In NSG mice treated with MRTX1133 (up to 30 mg/kg, oral, daily for 28 days), no adverse effects on hematological parameters (WBC, RBC, platelets) or kidney function (BUN, creatinine) are noted [3] - Plasma protein binding rate of MRTX1133 is 92.3 ± 2.1% (rat plasma) and 94.1 ± 1.8% (human plasma), determined by equilibrium dialysis [4] - No significant QT interval prolongation is observed in rats at doses up to 50 mg/kg/day [3] |
| 参考文献 |
|
| 其他信息 |
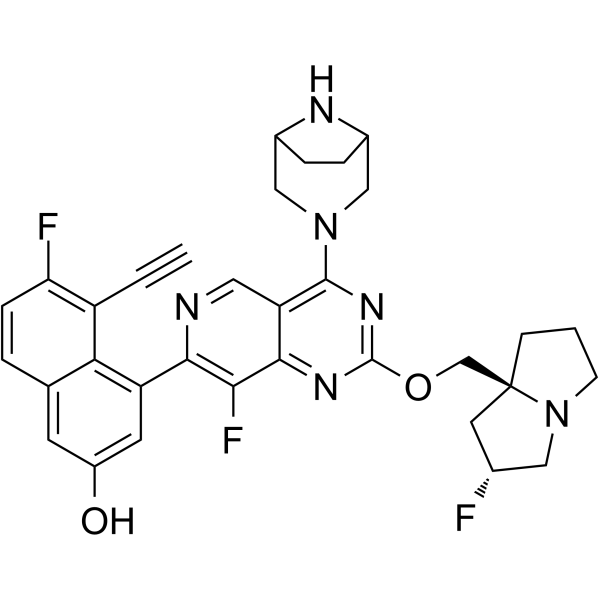
KRAS G12D Inhibitor MRTX1133 is an orally bioavailable reversible inhibitor of the oncogenic KRAS substitution mutation G12D, with potential antineoplastic activity. Upon oral administration, KRAS G12D inhibitor MRTX1133 specifically targets and noncovalently binds to KRAS G12D. This prevents KRAS G12D-mediated signaling and activation of downstream survival pathways. This leads to an inhibition of the growth of tumor cells that overexpress KRAS G12D. KRAS, a member of the RAS family of oncogenes, serves an important role in cell signaling, division and differentiation. Mutations of KRAS may induce constitutive signal transduction leading to tumor cell proliferation, invasion, and metastasis.\n
\n\nLung cancer, the leading cause of cancer-related deaths worldwide, can be classified into small cell lung cancer and non-small cell lung cancer (NSCLC). NSCLC is the most common histological type, accounting for 85% of all lung cancers. Kirsten rat sarcoma viral oncogene (KRAS) mutations, common in NSCLC, are associated with poor prognosis, likely due to poor responses to most systemic therapies and lack of targeted drugs. The latest published clinical trial data on new small-molecule KRAS G12C inhibitors, AMG510 and MRTX849, indicate that these molecules may potentially help treat KRAS-mutant NSCLC. Simultaneously, within the immuno-therapeutic process, immune efficacy has been observed in those patients who have KRAS mutations. In this article, the pathogenesis, treatment status, progress of immunotherapy, and targeted therapy of KRAS-mutant NSCLC are reviewed. [1] \n\nThrough extensive structure-based drug design, MRTX1133 was identified as a noncovalent, potent, and selective inhibitor of KRASG12D. MRTX1133 suppresses KRASG12D signaling in cells and in vivo, and its antitumor benefit was demonstrated in a murine animal model. To the best of our knowledge, this is the first report in the literature of a small molecule inhibitor of KRASG12D that exhibits robust in vivo efficacy. These data support the potential for the advancement of an effective therapeutic against this “undruggable” target. The optimization process was facilitated by high-resolution X-ray crystal structures. In-depth binding mode analysis derived from cocrystal structures allowed the optimization of lipophilic contact of the inhibitor in the binding pocket and the identification of nonclassical hydrogen bonding and ion pair interactions, ultimately increasing selective binding affinity for KRASG12D by more than 1,000,000-fold relative to the initial hit 5B. MRTX1133 binds to the switch II pocket and inhibits the protein–protein interactions necessary for activation of the KRAS pathway. MRTX1133 not only possesses single-digit nM potency in a cellular proliferation assay, but also demonstrates tumor regressions in the Panc 04.03 xenograft model. A more comprehensive in vitro and in vivo pharmacological characterization of MRTX1133 will be disclosed in due course.[2] \n\nKRASG12D, the most common oncogenic KRAS mutation, is a promising target for the treatment of solid tumors. However, when compared to KRASG12C, selective inhibition of KRASG12D presents a significant challenge due to the requirement of inhibitors to bind KRASG12D with high enough affinity to obviate the need for covalent interactions with the mutant KRAS protein. Here, we report the discovery and characterization of the first noncovalent, potent, and selective KRASG12D inhibitor, MRTX1133, which was discovered through an extensive structure-based activity improvement and shown to be efficacious in a KRASG12D mutant xenograft mouse tumor model. [2] \n\nThese data indicate that discovery and pre-clinical development of high-affinity, mutation-selective, non-covalent inhibitors of KRASG12D and perhaps other KRAS mutant variants is feasible. As KRASG12D is the most prevalent of KRAS mutant alleles, the translation of these findings to a reality for patients with cancer harboring KRASG12D mutations would be highly impactful. Additionally, the anticipated therapeutic index for allele-specific inhibitors may provide an advantage in both facilitating maximal target inhibition and a favorable combinatorial therapy profile. These studies also provide insight toward the function of this mutation as an oncogenic driver in different tumor types and in the context of co-occurring genetic alterations. The ability to also characterize the effect of MRTX1133 on KRAS-dependent signaling and feedback pathways using molecular profiling approaches and functional genomics helps increase understanding of unique aspects of KRASG12D signaling. In turn, the understanding of KRASG12D signaling dynamics provides rational perspective on co-targeting of collateral dependencies. Collectively, the present studies provide renewed perspective on direct targeting strategies for KRAS and provide defining strategies to identify patients likely to benefit from single-agent or rationally directed combinations.[3]\n \nThis study was the first to evaluate the pharmacokinetics, bioavailability, distribution, and excretion of MRTX1133 in rats. A sensitive, rapid, and reliable UHPLC-MS/MS method was developed to measure MTRX1133 in rat plasma, tissue homogenate and urine. The established method was successfully applied to the pharmacokinetic study of MTRX1133 in rats after administration by different routes. MRTX1133 is quickly absorbed after oral administration and widely distributed in the body. But the bioavailability is very low and only 24% of the drug were excreted through the kidneys by the original form. This study might provide a sufficient reference for the quantitative determination of MTRX1133, in preclinical or clinical studies/trials. MRTX1133 is a first-in-class, noncovalent, highly selective KRASG12D inhibitor designed for the treatment of KRASG12D-mutant solid tumors [2][3] - Its mechanism of action involves binding to the switch II pocket of KRASG12D, stabilizing the inactive GDP-bound state and blocking downstream RAS-MAPK and PI3K-AKT signaling pathways [2][3] - MRTX1133 demonstrates clinical potential for KRASG12D-mutant NSCLC, PDAC, and colorectal cancer, with synergistic activity when combined with EGFR inhibitors (cetuximab) [3] - KRASG12D is one of the most common KRAS mutations in solid tumors, accounting for ~44% of KRAS-mutant PDAC and ~14% of KRAS-mutant NSCLC [2] |
| 分子式 |
C33H31F3N6O2
|
|---|---|
| 分子量 |
600.6335
|
| 精确质量 |
600.246
|
| 元素分析 |
C, 65.99; H, 5.20; F, 9.49; N, 13.99; O, 5.33
|
| CAS号 |
2621928-55-8
|
| 相关CAS号 |
2621928-55-8;
|
| PubChem CID |
156124857
|
| 外观&性状 |
Yellow to brown solid powder
|
| LogP |
5.2
|
| tPSA |
86.6Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
1100
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C#CC1=C(C=CC2=CC(=CC(=C21)C3=NC=C4C(=C3F)N=C(N=C4N5CC6CCC(C5)N6)OC[C@@]78CCCN7C[C@@H](C8)F)O)F
|
| InChi Key |
SCLLZBIBSFTLIN-IFMUVJFISA-N
|
| InChi Code |
InChI=1S/C33H31F3N6O2/c1-2-23-26(35)7-4-18-10-22(43)11-24(27(18)23)29-28(36)30-25(13-37-29)31(41-15-20-5-6-21(16-41)38-20)40-32(39-30)44-17-33-8-3-9-42(33)14-19(34)12-33/h1,4,7,10-11,13,19-21,38,43H,3,5-6,8-9,12,14-17H2/t19-,20?,21?,33+/m1/s1
|
| 化学名 |
4-[4-(3,8-diazabicyclo[3.2.1]octan-3-yl)-8-fluoro-2-[[(2R,8S)-2-fluoro-1,2,3,5,6,7-hexahydropyrrolizin-8-yl]methoxy]pyrido[4,3-d]pyrimidin-7-yl]-5-ethynyl-6-fluoronaphthalen-2-ol
|
| 别名 |
MRTX1133; MRTX 1133; MRTX1,133; 2621928-55-8; 4-[4-(3,8-Diazabicyclo[3.2.1]oct-3-yl)-8-fluoro-2-[[(2R,7aS)-2-fluorotetrahydro-1H-pyrrolizin-7a(5H)-yl]methoxy]pyrido[4,3-d]pyrimidin-7-yl]-5-ethynyl-6-fluoro-2-naphthalenol; CHEMBL4858364; 4-(4-(3,8-Diazabicyclo[3.2.1]octan-3-yl)-8-fluoro-2-(((2R,7aS)-2-fluorohexahydro-1H-pyrrolizin-7a-yl)methoxy)pyrido[4,3-d]pyrimidin-7-yl)-5-ethynyl-6-fluoronaphthalen-2-ol; 4-[4-(3,8-diazabicyclo[3.2.1]octan-3-yl)-8-fluoro-2-[[(2R,8S)-2-fluoro-1,2,3,5,6,7-hexahydropyrrolizin-8-yl]methoxy]pyrido[4,3-d]pyrimidin-7-yl]-5-ethynyl-6-fluoronaphthalen-2-ol; MFCD34567005; MRTX-1133
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO:50~100 mg/mL (83.3~166.5 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 10 mg/mL (16.65 mM) in 10% SBE-β-CD/50 mM citrate pH 5.0 (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
配方 2 中的溶解度: 3.5 mg/mL (5.83 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 35.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.16 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO+40%PEG300+5%Tween80+50%ddH2O: 25mg/ml 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6649 mL | 8.3246 mL | 16.6492 mL | |
| 5 mM | 0.3330 mL | 1.6649 mL | 3.3298 mL | |
| 10 mM | 0.1665 mL | 0.8325 mL | 1.6649 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05737706 | Recruiting | Drug: MRTX1133 | Solid Tumor Advanced Solid Tumor |
Mirati Therapeutics Inc. | March 20, 2023 | Phase 1 Phase 2 |
|
|