| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
奈法唑酮会引起氧化应激(表现为谷胱甘肽耗尽)并扰乱线粒体膜的电位,从而导致细胞死亡[2]。在葡萄糖和半乳糖上培养的 HepG2 细胞在给予奈瓦佐酮 (200 μM) 24 小时后显示 100% ATP 耗尽 [2]。 Nevazodone(6.25、12.5 和 25 μM;0-120 分钟)可显着抑制 HepG2 耗氧量 (OCR) [2]。
|
|---|---|
| 体内研究 (In Vivo) |
Nefazodone (10 mg/kg) 在 16 天的时间内皮下注射,可有效抵消压力对小鼠免疫系统的有害影响 [3]。
|
| 动物实验 |
Animal/Disease Models: Female balb/c (Bagg ALBino) mouse (7-12 weeks old; stress model; exposed to 100 dB broadband noise daily for 5 seconds per minute for 1 or 3 hrs (hrs (hours)) around midnight) [3]
Doses: 10 mg/kg Route of Administration: subcutaneous injection; lasts for 16 days. Experimental Results: Reduces stress-induced decreases in the number of thymus, spleen and peripheral blood cells. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Nefazodone is rapidly and completely absorbed. Its absolute bioavailability is low (about 20%). Nefazodone is extensively metabolized after oral administration by n-dealkylation and aliphatic and aromatic hydroxylation, and less than 1% of administered nefazodone is excreted unchanged in urine. 0.22 to 0.87 L/kg /MILK/ In two ... subjects, one taking 50 mg twice daily and the other 50 mg in the morning and 100 mg in the evening, the trough plasma levels were <50 ng/mL, whereas the paired milk concentrations were 687 and 213 ng/mL, respectively. /MILK/ ... In one woman taking 200 mg twice daily /of nefazodone/, the paired concentrations of nefazodone in milk and plasma (trough) were 57 and 617 ng/mL, respectively. the milk:plasma ratio was 0.09. /MILK/ Nefazodone is excreted into breast milk. /MILK/ The purpose of this study was/ to investigate whether adverse effects in a premature neonate could be attributed to nefazodone exposure via breast milk. The breast-fed white infant (female, 2.1 kg, 36 weeks corrected gestational age) of a 35-year-old woman (60 kg) taking nefazodone 300 mg/day was admitted to the hospital because she was drowsy, lethargic, unable to maintain normal body temperature, and was feeding poorly. A diagnosis of exposure to nefazodone via breast milk was considered only after other more likely diagnoses had been excluded. ... The maternal plasma and milk concentration-time profiles for nefazodone and its metabolites, triazoledione, HO-nefazodone, and m-chlorphenylpiperazine, were quantified by HPLC. The calculated infant dose for nefazodone and its active metabolites (as nefazodone equivalents) via the milk was only 0.45% of the weight-adjusted maternal nefazodone daily dose. ... For more Absorption, Distribution and Excretion (Complete) data for Nefazodone (7 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. ... A 16-year-old female took 2.4 g of nefazodone. ... The terminal elimination half-life for nefazodone was 8.3 hours, and its metabolite hydroxy(OH)-nefazodone was 14.6 hours. BP-time curves demonstrated an 18-hour period of hypotension. There was a significant correlation between systolic BP and OH-nefazodone (R2 = 0.602). HR remained between 56 and 66 bpm for 30 hours despite hypotension. QT was significantly correlated with nefazodone (R2 = 0.911) and OH-nefazodone (R2 = 0.797), but no significant relationship between QTc and drug concentrations. ... The purpose of this study was/ to investigate whether adverse effects in a premature neonate could be attributed to nefazodone exposure via breast milk. The breast-fed white infant (female, 2.1 kg, 36 weeks corrected gestational age) of a 35-year-old woman (60 kg) taking nefazodone 300 mg/day was admitted to the hospital because she was drowsy, lethargic, unable to maintain normal body temperature, and was feeding poorly. ... The maternal plasma and milk concentration-time profiles for nefazodone and its metabolites, triazoledione, HO-nefazodone, and m-chlorphenylpiperazine, were quantified by HPLC. The calculated infant dose for nefazodone and its active metabolites (as nefazodone equivalents) via the milk was only 0.45% of the weight-adjusted maternal nefazodone daily dose. ... The utility of multivariate analysis in in vitro metabolite identification studies was examined with nefazodone, an antidepressant drug with a well-established metabolic profile. The chromatographic conditions were purposefully chosen to reflect those utilized in high-throughput screening for microsomal stability of new chemical entities. Molecular ion, retention time information on groups of human liver microsomal samples with/without nefazodone was evaluated by principal component analysis (PCA). Resultant scores and loadings plots from the PCA revealed the segregation and the ions of interest that designated the drug and its corresponding metabolites. Subsequent acquisition of tandem mass spectrometry (MS/MS) spectra for targeted ions permitted the interrogation and interpretation of spectra to identify nefazodone and its metabolites. A comparison of nefazodone metabolites identified by PCA versus those found by traditional metabolite identification approaches resulted in very good correlation when utilizing similar analytical methods. Fifteen metabolites of nefazodone were identified in beta-nicotinamide adenine dinucleotide phosphate (NADPH)-supplemented human liver microsomal incubations, representing nearly all primary metabolites previously reported. Of the 15 metabolites, eight were derived from the N-dealkylation and N-dephenylation of the N-substituted 3-chlorophenylpiperazine motif in nefazodone, six were derived from mono- and bis-hydroxylation, and one was derived from the Baeyer Villiger oxidation of the ethyltriazolone moiety in nefazodone. Nefazodone is extensively metabolized after oral administration by n-dealkylation and aliphatic and aromatic hydroxylation, and less than 1% of administered nefazodone is excreted unchanged in urine. Attempts to characterize three metabolites identified in plasma, hydroxynefazodone (HO-NEF), meta-chlorophenylpiperazine (mCPP), and a triazole-dione metabolite, have been carried out. This paper describes the complete profiling and characterization of in vitro metabolites of the antidepressant agent nefazodone (NEF) generated by human liver microsome (HLM). Two new metabolic pathways (biotransformation) for NEF have been discovered by the characterization of three new metabolites, including two new metabolites (M24, M25) formed due to the N-dealkylation reaction that occurred between the triazolone and propyl units, and one new metabolite (M26) formed due to the O-dearylation reaction that occurred on the phenoxyethyl unit. These metabolites were initially detected by a 4000 Q-Trap instrument and then confirmed by exact mass measurement using an LTQ-Orbitrap. Both instruments proved to be capable of providing complete in vitro metabolite information in a single liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis, although each had its advantages and disadvantages. One noticeable disadvantage of the 4000 Q-Trap was the reduced quality of isotopic pattern in the enhanced mass scan (EMS) spectrum when it was used as survey scan to trigger multiple dependent product ion scans. The problem was especially exacerbated for minor metabolites with low signal intensity. On the other hand, the LTQ-Orbitrap maintained excellent isotopic pattern when used as a full scan survey scan. Twenty-six metabolites were detected and identified. The formation of these new metabolites was also confirmed by analyzing duplicate incubations at different time points. Nefazodone has known human metabolites that include Hydroxynefazodone, 1-(3-Chlorophenyl)piperazine, 5-Ethyl-4-(2-phenoxyethyl)-2-(3-hydroxypropyl)-2H-1,2,4-triazol-3(4H)-one, and P-Hydroxynefazodone. Hepatic. Route of Elimination: Nefazodone is extensively metabolized after oral administration by n-dealkylation and aliphatic and aromatic hydroxylation, and less than 1% of administered nefazodone is excreted unchanged in urine. Half Life: 2-4 hours Biological Half-Life 2-4 hours ... A 16-year-old female took 2.4 g of nefazodone. ... The terminal elimination half-life for nefazodone was 8.3 hours, and its metabolite hydroxy(OH)-nefazodone was 14.6 hours. ... In a 4-way crossover design, 16 subjects received clinically relevant doses of venlafaxine, nefazodone, or sertraline for 8 days or fluoxetine for 11 days. Treatments were separated by a 7- to 14-day washout period and fluoxetine was always the last antidepressant taken. ... Nefazodone was also the only antidepressant that caused a significant change in alprazolam (ALPZ) disposition, decreasing its area under the concentration-versus-time curve (AUC; P < 0.01), and increasing its elimination half-life (16.4 vs. 12.3 hours; P < 0.05) compared with values at baseline. ... ... the half-life of nefazodone is 2 to 4 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Nefazodone is a solid. It is used as an antidepressive agent. HUMAN STUDIES: In postmarketing experience, overdose with nefazodone alone and in combination with alcohol and/or other substances has been reported. While there have been rare reports of fatalities in patients taking overdoses of nefazodone, predominantly in combination with alcohol and/or other substances, no causal relationship to nefazodone has been established. In premarketing clinical studies, there were seven reports of nefazodone overdose alone or in combination with other pharmacological agents. None of these patients died. The amount of nefazodone ingested ranged from 1000 mg to 11,200 mg. Commonly reported symptoms from overdose of nefazodone included nausea, vomiting, and somnolence. One patient took 2000 to 3000 mg of nefazodone with methocarbamol and alcohol, and this person reportedly experienced a convulsion. Nefazodone therapy has been associated with liver abnormalities ranging from asymptomatic reversible serum transaminase increases to cases of liver failure resulting in transplant and/or death. At present, there is no way to predict who is likely to develop liver failure. Ordinarily, patients with active liver disease should not be treated with nefazodone. The drug does not increase the rates of major human malformations during pregnancy above the baseline rate of 1% to 3%. ANIMAL STUDIES: There is no evidence of carcinogenicity with nefazodone. The dietary administration of nefazodone to rats and mice for 2 years at daily doses of up to 200 mg/kg and 800 mg/kg, respectively, produced no increase in tumors. Reproduction studies have been performed in pregnant rabbits and rats at daily doses up to 200 and 300 mg/kg, respectively (approximately 6 and 5 times, respectively. No malformations were observed in the offspring as a result of nefazodone treatment. However, increased early pup mortality, and decreased pup weights were seen in rats. A fertility study in rats showed a slight decrease in fertility at 200 mg/kg/day. Nefazodone has been shown to have no genotoxic effects based on the following assays: bacterial mutation assays, a DNA repair assay in cultured rat hepatocytes, a mammalian mutation assay in Chinese hamster ovary cells, an in vivo cytogenetics assay in rat bone marrow cells, and a rat dominant lethal study. Within the serotonergic system, nefazodone acts as an antagonist at type 2 serotonin (5-HT2) post-synaptic receptors and, like fluoxetine-type antidepressants, inhibits pre-synaptic serotonin (5-HT) reuptake. These mechanisms increase the amount of serotonin available to interact with 5-HT receptors. Within the noradrenergic system, nefazodone inhibits norepinephrine uptake minimally. Nefazodone also antagonizes alpha(1)-adrenergic receptors, producing sedation, muscle relaxation, and a variety of cardiovascular effects. Nefazodone's affinity for benzodiazepine, cholinergic, dopaminergic, histaminic, and beta or alpha(2)-adrenergic receptors is not significant. Interactions Simvastatin is a hydroxymethyl glutaryl coenzyme A reductase inhibitor commonly used to treat patients with hyperlipidemia. It is a safe and effective medication in most patients when used appropriately. A serious side effect known as rhabdomyolysis may rarely occur in patients who take simvastatin, especially at higher doses and with agents that interact and increase the level of simvastatin in the blood. We describe the case of a patient with rhabdomyolysis that occurred after the patient's simvastatin was titrated to 80 mg at approximately the same time that his antidepressant medication was switched to nefazodone. We found only two other similar cases in the literature, both of which were presented as letters to the editor in two different journals. We present this case to add to the literature and to assist practitioners by raising their awareness of this interaction so that it can be monitored. This study was conducted to determine the potential for an interaction between nefazodone, a new antidepressant, and alprazolam after single- and multiple-dose administration in a randomized, double-blind, parallel-group, placebo-controlled study in 48 healthy male volunteers. A group of 12 subjects received either placebo twice daily, 1 mg of alprazolam twice daily, 200 mg of nefazodone twice daily, or the combination of 1 mg of alprazolam and 200 mg of nefazodone twice daily for 7 days. Serial blood samples were collected after dosing on day 1 and day 7 and before the morning dose on days 4, 5, and 6 for the determination of alprazolam and its metabolites alpha-hydroxyalprazolam (AOH) and 4-hydroxyalprazolam (4OH) and nefazodone and its metabolites hydroxynefazodone (HO-nefazodone), m-chlorophenylpiperazine (mCPP), and a triazole dione metabolite (dione) by validated high-performance liquid chromatography methods. Steady-state levels in plasma were reached by day 4 for alprazolam, 4OH, nefazodone, HO-nefazodone, mCPP, and dione. Noncompartmental pharmacokinetic analysis showed that at steady state, alprazolam Cmax and AUCtau values significantly increased approximately twofold and 4OH Cmax and AUCtau values significantly decreased by 40 and 26%, respectively, when nefazodone was coadministered with alprazolam. There was no effect of alprazolam on the single-dose or steady-state pharmacokinetics of nefazodone, HO-nefazodone, or dione after the coadministration of alprazolam and nefazodone. However, the mean steady-state mCPP Cmax and AUCtau significantly increased by approximately threefold and t1/2 values significantly increased by approximately twofold after the coadministration of alprazolam and nefazodone in comparison to those when nefazodone was given alone. Competitive inhibition between alprazolam and nefazodone metabolism at cytochrome P450 3A4 may be responsible for the pharmacokinetic interaction when alprazolam and nefazodone were coadministered. No adjustment of nefazodone dosage is required when nefazodone and alprazolam are coadministered. Because alprazolam concentrations in plasma are increased in the presence of nefazodone, a reduction in alprazolam dosage is recommended when the two agents are coadministered. Tacrolimus (FK-506) is an important immunosuppressive agent most often given for maintenance immunosuppression to prevent acute cellular organ rejection. A 57-year-old woman with end-stage renal disease presumed secondary to chronic glomerulonephritis underwent a living related renal allograft transplantation. She tolerated the surgery well and was discharged on postoperative day 5. She was stabilized with prednisone, azathioprine, and tacrolimus. Two years after transplantation, nefazodone 50 mg twice/day orally was prescribed due to depression. After 1 week of nefazodone therapy the patient experienced headache, confusion, and "gray areas" in her vision, without abnormal ophthalmologic findings. Her serum creatinine was elevated to 2.2 mg/dL (baseline 1.5 mg/dL), and trough tacrolimus level was markedly elevated (> 30 ng/mL). Both tacrolimus and nefazodone are metabolized by the cytochrome P450 (CYP) 3A4 system. We suspect that nefazodone inhibits metabolism of tacrolimus. Coadministration of antidepressant agents such as nefazodone, or any other drug that inhibits the CYP3A4 isoenzyme subfamily, should be anticipated to interfere with tacrolimus metabolism. Monitoring blood concentrations of tacrolimus is vital, and appropriate dosage adjustments are required when the two drugs are administered concurrently to avoid serious interactions such as nephrotoxicity and neurotoxicity. Depression is a significant post-transplant complication often necessitating drug therapy. Many of the newer selective serotonin reuptake inhibitor (SSRI) antidepressants are metabolized by the same cytochrome P450IIIA isoenzyme system that is responsible for the metabolism of cyclosporine, and these agents pose an interactive risk in transplant patients. We have observed nearly a 10-fold increase in whole blood cyclosporine concentrations in a cardiac transplant patient shortly after the addition of nefazodone antidepressant therapy. We suggest there is a clinically significant drug-drug interaction between nefazodone and cyclosporine due to inhibition of cytochrome P-450 IIIA4 isoenzymes by nefazodone. For more Interactions (Complete) data for Nefazodone (26 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Nefazodone is a N-arylpiperazine, a N-alkylpiperazine, a member of triazoles, a member of monochlorobenzenes and an aromatic ether. It has a role as an antidepressant, a serotonergic antagonist, a serotonin uptake inhibitor, an alpha-adrenergic antagonist and an analgesic.
Nefazodone hydrochloride (trade name Serzone) is an antidepressant drug marketed by Bristol-Myers Squibb. Its sale was discontinued in 2003 in some countries, due to the small possibility of hepatic (liver) injury. Drug-induced hepatic injuries were associated with an risk of elevated need for a liver transplant, or even death, with the incidence of severe liver damage was shown to be approximately 1 in 250,000 to 300,000 patient-years. On May 20, 2004, Bristol-Myers Squibb discontinued the sale of Serzone in the United States. Nefazodone is a Serotonin Reuptake Inhibitor. Nefazodone is a serotoninergic modulating antidepressant that is used in therapy of depression, aggressive behavior and panic disorder. Nefazodone therapy has been associated with transient, usually asymptomatic elevations in serum aminotransferase levels and has been linked to several instances of clinically apparent acute liver injury some of which have been fatal. Nefazodone hydrochloride (trade name Serzone) is an antidepressant drug marketed by Bristol-Myers Squibb. Its sale was discontinued in 2003 in some countries, due to the small possibility of hepatic (liver) injury, which could lead to the need for a liver transplant, or even death. The incidence of severe liver damage is approximately 1 in 250,000 to 300,000 patient-years. On May 20, 2004, Bristol-Myers Squibb discontinued the sale of Serzone in the United States. [Wikipedia] See also: Nefazodone Hydrochloride (has salt form). Drug Indication For the treatment of depression. FDA Label Mechanism of Action Within the serotonergic system, nefazodone acts as an antagonist at type 2 serotonin (5-HT2) post-synaptic receptors and, like fluoxetine-type antidepressants, inhibits pre-synaptic serotonin (5-HT) reuptake. These mechanisms increase the amount of serotonin available to interact with 5-HT receptors. Within the noradrenergic system, nefazodone inhibits norepinephrine uptake minimally. Nefazodone also antagonizes alpha(1)-adrenergic receptors, producing sedation, muscle relaxation, and a variety of cardiovascular effects. Nefazodone's affinity for benzodiazepine, cholinergic, dopaminergic, histaminic, and beta or alpha(2)-adrenergic receptors is not significant. Therapeutic Uses Antidepressive Agents, Second-Generation /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Nefazodone is included in the database. Nefazodone hydrochloride tablets are indicated for the treatment of depression. When deciding among the alternative treatments available for this condition, the prescriber should consider the risk of hepatic failure associated with nefazodone hydrochloride treatment. In many cases, this would lead to the conclusion that other drugs should be tried first. /Included in US product label/ Drug Warnings /BOXED WARNING/ Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of nefazodone hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Nefazodone hydrochloride tablets are not approved for use in pediatric patients. /BOXED WARNING/ Cases of life-threatening hepatic failure have been reported in patients treated with nefazodone hydrochloride tablets. The reported rate in the United States is about 1 case of liver failure resulting in death or transplant per 250,000 to 300,000 patient-years of nefazodone hydrochloride treatment. The total patient-years is a summation of each patient's duration of exposure expressed in years. For example, 1 patient-year is equal to 2 patients each treated for 6 months, 3 patients each treated for 4 months, etc. Ordinarily, treatment with nefazodone hydrochloride tablets should not be initiated in individuals with active liver disease or with elevated baseline serum transaminases. There is no evidence that pre-existing liver disease increases the likelihood of developing liver failure, however, baseline abnormalities can complicate patient monitoring. Patients should be advised to be alert for signs and symptoms of liver dysfunction (jaundice, anorexia, gastrointestinal complaints, malaise, etc.) and to report them to their doctor immediately if they occur. Nefazodone hydrochloride tablets should be discontinued if clinical signs or symptoms suggest liver failure. Patients who develop evidence of hepatocellular injury such as increased serum AST or serum ALT levels >/= 3 times the upper limit of NORMAL, while on nefazodone hydrochloride tablets should be withdrawn from the drug. These patients should be presumed to be at increased risk for liver injury if nefazodone hydrochloride is reintroduced. Accordingly, such patients should not be considered for re-treatment. The most commonly observed adverse events associated with the use of nefazodone (incidence of 5% or greater) and not seen at an equivalent incidence among placebo-treated patients (i.e., significantly higher incidence for nefazodone compared to placebo, p Approximately 16% of the 3496 patients who received nefazodone in worldwide premarketing clinical trials discontinued treatment due to an adverse experience. The more common (>/= 1%) events in clinical trials associated with discontinuation and considered to be drug related (i.e., those events associated with dropout at a rate approximately twice or greater for nefazodone compared to placebo) included: nausea (3.5%), dizziness (1.9%), insomnia (1.5%), asthenia (1.3%), and agitation (1.2%). For more Drug Warnings (Complete) data for Nefazodone (17 total), please visit the HSDB record page. Pharmacodynamics Nefazodone, an antidepressant synthetically derived phenylpiperazine, is used to treat major depression. Although it is structurally similar to trazodone, nefazodone has a mechanism of action different from other antidepressants and, hence, lacks the risk for major cardiovascular toxicity seen with tricyclics and insomnia and inhibition of REM sleep seen with the selective serotonin reuptake inhibitors. |
| 分子式 |
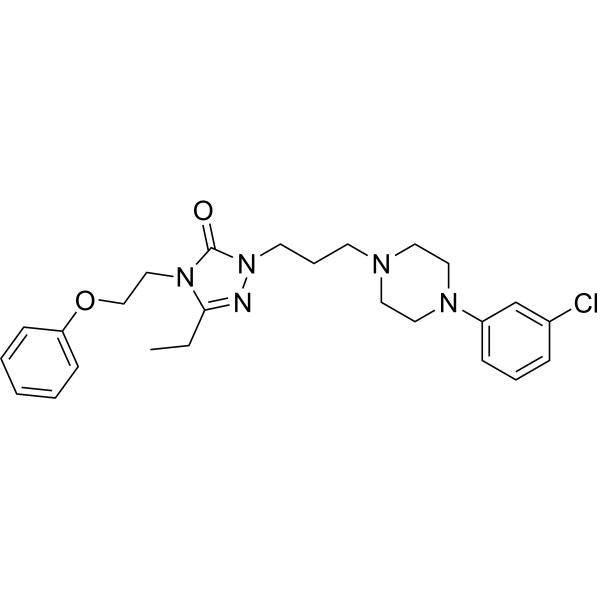
C25H32N5O2CL
|
|---|---|
| 分子量 |
470.00688
|
| 精确质量 |
469.224
|
| 元素分析 |
C, 63.89; H, 6.86; Cl, 7.54; N, 14.90; O, 6.81
|
| CAS号 |
83366-66-9
|
| 相关CAS号 |
Nefazodone hydrochloride;82752-99-6
|
| PubChem CID |
4449
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 熔点 |
180-182°C
|
| LogP |
3.554
|
| tPSA |
55.53
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
649
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCC1=NN(CCCN2CCN(CC2)C2=CC(Cl)=CC=C2)C(=O)N1CCOC1=CC=CC=C1
|
| InChi Key |
VRBKIVRKKCLPHA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3
|
| 化学名 |
2-[3-[4-(3-chlorophenyl)piperazin-1-yl]propyl]-5-ethyl-4-(2-phenoxyethyl)-1,2,4-triazol-3-one
|
| 别名 |
Nefazodone, Dutonin, Nefadar, Serzone
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~265.95 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1276 mL | 10.6381 mL | 21.2761 mL | |
| 5 mM | 0.4255 mL | 2.1276 mL | 4.2552 mL | |
| 10 mM | 0.2128 mL | 1.0638 mL | 2.1276 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。