| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
HER2 (IC50 = 59 nM); EGFR (IC50 = 92 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Neratinib 弱抑制酪氨酸激酶 KDR 和 Src,IC50 分别为 0.8 μM 和 1.4 μM,与 HER2 相比活性低 14 倍和 24 倍。 Neratinib 对其他丝氨酸-苏氨酸激酶没有活性,例如 Akt、细胞周期蛋白 D1/cdk4、细胞周期蛋白 E/cdk2、细胞周期蛋白 B1/cdk1、IKK-2、MK-2、PDK1、c-Raf 和 Tpl-2。如酪氨酸激酶 c-Met。 Neratinib 选择性抑制转染 HER2 (3T3/neu) 的 3T3 细胞以及另外两种 HER-2 过表达 SK-Br-3 和 BT474 细胞的增殖,IC50 值为 2-3 nM,显示 >230 倍与未转染的 3T3 细胞以及 EGFR 和 HER2 阴性的 MDA-MB-435 和 SW620 细胞相比,其效力较高。 Neratinib 还抑制 EGFR 依赖性 A431 细胞的增殖,IC50 为 81 nM。 Neratinib 可减少 BT474 细胞中 HER2 受体的自身磷酸化,IC50 为 5 nM,并减少 A431 细胞中 EGFR 的 EGF 依赖性磷酸化,IC50 为 3 nM。 Neratinib 阻断 HER-2 导致下游 MAPK 和 Akt 通路的抑制,IC50 为 2 nM,比曲妥珠单抗更有效。 Neratinib 抑制 BT474 细胞中细胞周期蛋白 D1 的表达和 Rb 易感基因产生的磷酸化,IC50 为 9 nM,导致 G1-S 停滞并最终降低细胞增殖。激酶测定:Neratinib 在 DMSO 中制备为 10 mg/mL 库存,并在 25 mM HEPES(pH 7.5;0.002 ng/mL-20 μg/mL)中稀释。将 HER2(氨基酸 676-1255)或表皮生长因子受体 (EGFR)(氨基酸 645-1186)的纯化重组 COOH 末端片段 [用 100 mM HEPES (pH 7.5) 和 50% 甘油稀释] 进行浓度递增的培养将 Neratinib 溶解在 4 mM HEPES (pH 7.5)、0.4 mM MnCl2、20 μM 钒酸钠和 0.2 mM DTT 中,在 96 孔 ELISA 板中室温反应 15 分钟。通过添加 40 μM ATP 和 20 mM MgCl2 启动激酶反应,并在室温下进行 1 小时。洗涤板,并使用铕标记的抗磷酸酪氨酸抗体(15 ng/孔)检测磷酸化。洗涤和增强步骤后,使用 Victor2 荧光读数器(激发波长 340 nm,发射波长 615 nm)检测信号。根据抑制曲线计算抑制受体磷酸化 50% 的 Neratinib 浓度 (IC50)。细胞测定:将细胞(3T3、3T3/neu、A431、BT474、SK-Br-3、MDA-MB-435 和 SW480)暴露于不同浓度的 Neratinib 中 2 或 6 天。使用磺基罗丹明 B(一种蛋白质结合染料)测定细胞增殖。简而言之,用 10% 三氯乙酸固定细胞并用水充分洗涤。然后用 0.1% 磺基罗丹明 B 染色细胞并用 5% 乙酸洗涤。蛋白质相关染料溶解在 10 mM Tris 中,并在 450 nM 处测量吸光度。根据抑制曲线确定抑制细胞增殖 50% 的 Neratinib 浓度 (IC50)。
|
| 体内研究 (In Vivo) |
口服 Neratinib 可显着抑制 3T3/neu 异种移植物的生长,剂量为 10、20、40 和 80 mg/kg/天时,抑制率分别为 34%、53%、98% 和 98%。与 40 mg/kg/天给药 1 小时内 HER-2 磷酸化抑制 84% 一致,Neratinib 在 5、10 剂量时抑制 BT474 异种移植物的生长 70-82%、67% 和 93%和 40 毫克/公斤/天,分别。 Neratinib 对 SK-OV-3 异种移植物也有效,5 mg/kg/天和 60 mg/kg/天的抑制率分别为 31% 和 85%。 Neratinib 对 EGFR 依赖性 A431 异种移植物的效力低于 HER-2 依赖性肿瘤,在 5 和 20 mg/kg/天时分别具有 32% 和 44% 的抑制作用。 Neratinib 对表达低水平 HER-2 和 EGFR 的 MCF-7 和 MX-1 异种移植物几乎没有活性,在 80 mg/kg/天时仅抑制 28%,表明 Neratinib 对表达 HER-2 或 EGFR 的细胞具有选择性活性。
|
| 酶活实验 |
将来那替尼在 DMSO 中制备成 10 mg/mL 储备液,在 25 mM HEPES(pH 7.5;0.002 ng/mL–20 μg/mL)中稀释。在 96 孔 ELISA 板中,将 HER2(氨基酸 676-1255)或表皮生长因子受体(EGFR)(氨基酸 645-1186)的纯化重组 COOH 末端片段稀释在 100 mM HEPES(pH 7.5)和 50% 中。甘油。然后将混合物与浓度递增的 Neratinib 在 4 mM HEPES (pH 7.5)、0.4 mM MnCl2、20 μM 钒酸钠和 0.2 mM DTT 中在室温下孵育 15 分钟。添加 40 μM ATP 和 20 mM MgCl2 以启动激酶反应,然后在室温下运行一小时。清洗板,然后使用铕标记的抗磷酸酪氨酸抗体(15 ng/孔)检测磷酸化。使用 Victor2 荧光读数器(激发波长为 340 nm,发射波长为 615 nm),在洗涤和增强阶段后检测信号。抑制曲线用于确定受体磷酸化被抑制 50% 时 Neratinib 的浓度 (IC50)。
HER-2和EGFR细胞质结构域的活性通过时间分辨荧光法的自磷酸化测定来测量。化合物在DMSO中制备为10mg/ml储备,并在25mm HEPES(pH 7.5;0.002ng/ml–20μg/ml)中稀释。酶[在100 mm HEPES(pH 7.5)和50%甘油中稀释]在96孔ELISA板中与4 mm HEPES中的抑制剂、0.4 mm MnCl2、20μm钒酸钠和0.2 mm DTT在室温下孵育15分钟。通过加入40μm ATP和20mm MgCl2引发激酶反应,并在室温下进行1小时。洗涤板,使用铕标记的抗磷酸酪氨酸抗体(15ng/孔;Wallac)检测磷酸化。根据制造商的建议进行洗涤和增强步骤后,使用Victor2荧光阅读器(激发波长340nm,发射波长615nm)检测信号。根据抑制曲线计算抑制受体磷酸化50%的化合物浓度(IC50)。[1] 使用在细菌、昆虫或人类细胞系中表达的重组酶进行其他激酶的检测。除c-Met、KDR、src(酪氨酸激酶)和MEK1(双特异性)外,所有使用的酶都是丝氨酸-苏氨酸激酶。使用的底物是肽(Akt、IKK-2、MK2、PDK1、src和Tpl2)、蛋白质(细胞周期蛋白D1/CDK4、细胞周期蛋白E/CDK2、细胞周期素B1/CDK1和c-Raf)、聚谷氨酸4酪氨酸(KDR)或激酶本身(自磷酸化;c-met)。使用TMB过氧化物酶底物测量细胞周期蛋白/细胞周期蛋白依赖性激酶(cdk)的磷酸化,使用LabChip测量MK-2,或使用DELPHIA/LANCE 测量所有其他激酶的磷酸化。 |
| 细胞实验 |
将不同浓度的 Neratinib 应用于细胞(3T3、3T3/neu、A431、BT474、SK-Br-3、MDA-MB-435 和 SW480),持续两天或六天。一种称为磺基罗丹明 B 的蛋白质结合染料用于测量细胞增殖。总之,细胞用10%三氯乙酸固定后用水彻底清洗。用 0.1% 磺基罗丹明 B 染色细胞后,用 5% 乙酸冲洗。将蛋白质相关染料溶解在 10 mM Tris 中后,在 450 nM 处计算吸光度。抑制曲线用于计算neratinib抑制50%细胞增殖的浓度(IC50)。
|
| 动物实验 |
Female athymic (nude) mice, tumor xenograft[1]
10, 20, 40, 60 or 80 mg/kg/day Gavage, 42 days Tumor Xenograft Studies.[1] Tumor cells (maintained in tissue culture) or tumor fragments were implanted s.c. in the flanks of female athymic (nude) mice. For estrogen-dependent cell lines (BT474, MCF-7, and SK-OV-3), animals were implanted with hormone pellets (0.72 mg of 17-β estradiol, 60-day release) 1 week before implantation of tumors. Additionally, SK-OV-3 cells were suspended in Matrigel basement membrane matrix for implantation. Treatment was initiated after tumors had reached a size of 90–200 mg, following random assignment of the animals to different treatment groups (staging, day 0). For 3T3/neu xenografts, treatment was initiated the day after tumor implantation (day 0). HKI-272 was formulated in 0.5% methocellulose-0.4% polysorbate-80 (Tween 80) and administered daily, p.o., by gavage. Tumor mass [(length × width2)/2] was determined every 7 days. Tumor outgrowth in all xenograft studies, except 3T3/neu, was expressed as relative tumor growth: the ratio of the mean tumor mass to the mean tumor mass on day 0. Inhibition of tumor growth was calculated relative to vehicle-treated controls. Statistical significance of inhibition was demonstrated using one-tailed Student’s t test (equal variance) after log transformation of the data.[1] HER-2 Phosphorylation in Xenografts.[1] Athymic female nude mice (5 animals/group) were implanted s.c. with BT474 tumor fragments (∼30 mm3). When tumors reached 200–300 mg, animals were given a single oral dose (40 mg/kg) of HKI-272 in pH 2.0 water. Tumors from control and treated animals were excised at 1, 3, 6, and 24 h and minced. Tumor fragments were suspended in 10 mm Tris (pH 7.5), 5 mm EDTA, 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 2 mm sodium vanadate, and 100 mm sodium fluoride and lysed by homogenization on ice with a polytron. After clarification by centrifugation, protein concentration in lysates was estimated using the Bio-Rad DC protein assay. Sixty μg of lysate pooled from each group were analyzed by SDS-PAGE and immunoblotting with phospho-tyrosine-specific antibodies. Pooled extracts were also immunoprecipitated using 4 μg of anti-HER-2 antibodies for 1 h at 4°C. Immune complexes were collected on protein A-agarose, washed, and analyzed by immunoblotting using phospho-tyrosine-specific antibodies. Extracts from individual tumors were analyzed to determine variability between animals. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of neratanib during breastfeeding. Because neratanib and its metabolite are over 99% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during neratanib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
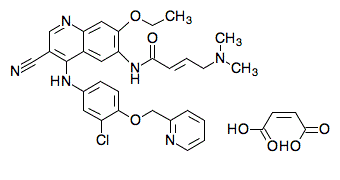
Neratinib Maleate is the maleate salt form of neratinib, an orally available, quinazoline-based, irreversible inhibitor of both the receptor tyrosine kinases (RTKs) human epidermal growth factor receptor 2 (HER2; ERBB2) and human epidermal growth factor receptor (EGFR), with potential antineoplastic activity. Upon administration, neratinib targets and covalently binds to the cysteine residue in the ATP-binding pockets of both HER2 and EGFR. This inhibits their activity and results in the inhibition of downstream signal transduction events, induces cell cycle arrest, apoptosis and ultimately decreases cellular proliferation in HER2- and EGFR-expressing tumor cells. EGFR and HER2, RTKs that are mutated or overactivated in many tumor cell types, play key roles in tumor cell proliferation and tumor vascularization.
See also: Neratinib (has active moiety). Drug Indication Nerlynx is indicated for the extended adjuvant treatment of adult patients with early stage hormone receptor positive HER2-overexpressed/amplified breast cancer and who are less than one year from the completion of prior adjuvant trastuzumab based therapy. |
| 分子式 |
C34H33CLN6O7
|
|---|---|
| 分子量 |
673.11
|
| 精确质量 |
672.209
|
| 元素分析 |
C, 60.67; H, 4.94; Cl, 5.27; N, 12.49; O, 16.64
|
| CAS号 |
915942-22-2
|
| 相关CAS号 |
Neratinib;698387-09-6
|
| PubChem CID |
67307512
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
187
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
48
|
| 分子复杂度/Complexity |
1000
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C(/C(=O)O)=C/C(=O)O.N(C1C=CC(OCC2N=CC=CC=2)=C(Cl)C=1)C1=C(C#N)C=NC2=CC(=C(C=C12)NC(=O)/C=C/CN(C)C)OCC
|
| InChi Key |
VXZCUHNJXSIJIM-MEBGWEOYSA-N
|
| InChi Code |
InChI=1S/C30H29ClN6O3.C4H4O4/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22;5-3(6)1-2-4(7)8/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38);1-2H,(H,5,6)(H,7,8)/b9-7+;2-1-
|
| 化学名 |
(Z)-but-2-enedioic acid;(E)-N-[4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide
|
| 别名 |
HKI-272 maleate or PB272; HKI272; 915942-22-2; hki-272 maleate; Nerlynx; Neratinib maleate [MI]; UNII-9RM7XY23ZS; 9RM7XY23ZS; Neratinib (maleate); HKI 272; PB 272; PB-272 maleate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4856 mL | 7.4282 mL | 14.8564 mL | |
| 5 mM | 0.2971 mL | 1.4856 mL | 2.9713 mL | |
| 10 mM | 0.1486 mL | 0.7428 mL | 1.4856 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06083662 | Active Recruiting |
Drug: Neratinib Maleate | Metastatic Cancer HER2 Gene Mutation |
Korea University Guro Hospital | June 15, 2021 | Phase 2 |
|
|---|
|
|