| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
p110α (Ki = 0.019 nM); p110α-E545K (Ki = 0.008 nM); p110α-E542K (Ki = 0.008 nM); p110α-H1047R (Ki = 0.009 nM); p110β (Ki = 0.13 nM); p110δ (Ki = 0.024 nM); p110γ (Ki = 0.06 nM); mTORC1 (Ki = 0.18 nM); mTORC2 (Ki = 0.3 nM)
|
|---|---|
| 体外研究 (In Vitro) |
GSK2126458 有效抑制人类癌症中常见的 p110α 激活突变体(E542K、E545K 和 H1047R)的活性,Ki 分别为 8 pM、8 pM 和 9 pM。 [1] GSK2126458 显着降低 T47D 和 BT474 细胞中 pAkt-S473 的水平,具有显着的效力,IC50 值分别为 0.41 nM 和 0.18 nM。此外,GSK2126458 会导致 G1 细胞周期停滞,并对多种细胞系(包括 T47D 和 BT474 乳腺癌系)的细胞增殖具有抑制作用,IC50 值分别为 3 nM 和 2.4 nM。 [1]
这些研究最终确定了Omipalisib/1,这是一种非常有效的PI3Kα抑制剂(p110α/p85α),具有低皮摩尔活性(PI3KαIC50=0.04 nM)。在生化检测中,化合物1的效力明显强于化合物2(PI3KαIC50=2 nM),是迄今为止报道的最有效的PI3Kα抑制剂。与其他临床PI3K抑制剂相比,1的效力比BEZ235(IC50=6 nM)和GDC-0941(IC50=9 nM)高约100倍,活性比XL-765(IC50=39 nM)低约1000倍。重要的是,1也是人类癌症中发现的p110α(E542K、E545K和H1047R)常见激活突变体的低皮摩尔抑制剂(表3)。与其他报道的PI3K抑制剂类似,1也对其他I类PI3K亚型(β、γ和δ)具有活性。[1] 化合物1/Omipalisib对蛋白激酶显示出优异的选择性(评估的激酶>10000倍对>240种),但IV类PI3K家族除外。mTOR是一种IV类PI3K蛋白激酶,是细胞生长的中枢调节因子,存在于两种功能复合物mTORC1和mTORC2中。(25)mTORC2被认为可以调节AKT S473磷酸化,其抑制作用被认为可以通过双重抑制PI3K/AKT途径来增强PI3K抑制剂的抗增殖功效。mTOR的激酶结构域与I类PI3K的p110α催化亚基同源,1是两种具有亚纳米活性的mTOR复合物的强效抑制剂(表4)。化合物1也是IV类PI3激酶DNA-PK的强效抑制剂(IC50=0.28 nM)。[1] PI3Kγ与1/Omipalisib复合物的共晶结构显示抑制剂结合在酶的ATP结合位点(图3)。该结构的分辨率为2.7Å,与6e一样,表明吡啶基氮与保守的水分子形成了一个关键的氢键。磺酰胺与Lys833相互作用,产生强烈的带电相互作用。根据磺酰胺NH的pKa(6.56),87%的部分在生理pH下以脱质子化形式存在。这种带电相互作用可能有助于解释1与其他报道的PI3K抑制剂相比具有更高的效力。此外,二氟苯基填充了酶后袋中的疏水区域,而喹啉氮与铰链形成相互作用(Val882)。[1] 在机械细胞试验中,1/Omipalisib导致pAKT-S473水平显著降低,具有显著效力(表5)。与它对PI3Kα和mTOR的活性一致,1在低纳摩尔浓度下也抑制AKT-T308和p70S6K的磷酸化(数据未显示)。化合物1在包括T47D和BT474乳腺癌症系的大量细胞系中诱导G1细胞周期阻滞并抑制细胞增殖。[1] 在四种临床前物种(小鼠、大鼠、狗和猴子)中研究了1/Omipalisib的PK谱。该化合物显示出较低的血液清除率和良好的口服生物利用度(表6)。此外,1抑制人细胞色素P450亚型的潜力很小(IC50>25μM,与CYP 3A4、1A2、2C9、2C19和2D6相比)。[1] 抑制MAPK和PI3K/mTOR通路可增强获得性耐药克隆的细胞生长抑制[2] 尽管GSK2118436和GSK1120212的组合显著抑制了抗性克隆的增殖,但S6P的磷酸化并没有被完全抑制。由于S6P磷酸化可以通过激活PI3K/mTOR途径诱导,我们评估了GSK2118436或GSK1120212与Omipalisib/GSK2126458(PI3K/m TOR抑制剂)的组合。所有克隆均显示出GSK2126458对细胞生长的适度敏感性抑制(表1)。代表性抗性克隆的GSK2126458处理降低了AKT磷酸化,对S6P磷酸化的影响很小(图5A)。Omipalisib/GSK2126458与GSK2118436或GSK1120212联合使用可降低抗性克隆中的S6P磷酸化,而单独使用GSK2118443或GSK1120 212足以降低A375中的S6P磷酸化。GSK2118436或GSK1120212与Omipalisib/GSK2126458组合对pS6P的减少大于GSK2118443和GSK1120212组合观察到的减少。MEK和ERK磷酸化与单独用GSK2118436或GSK1120212处理相似。在克隆16R6-4和16R6-2中,GSK2126458与GSK2118436或GSK1120212联合使用,切割的PARP和caspase-3/7活性(凋亡指标)略有增加,尽管与A375相比,所有抗性克隆的基础凋亡水平都更高(图5A和数据未显示)。在GSK2126458中添加GSK2118436增强了5/7个NRAS突变克隆和2个携带MEK1K59del克隆的细胞生长抑制(EOSHA>10 ppts)(表1)。GSK1120212和GSK2126458的组合在8/9个克隆中具有协同作用(CI<0.8),在所有9个克隆(无论NRAS或MEK1突变如何)中都增强了细胞生长抑制(EOSHA>20 ppts)(表1)。长期增殖试验证实,GSK2118436或GSK1120212与GSK2126458的组合增强了生长抑制作用(图5B)。一般来说,抗性克隆对所用浓度的GSK2126458和GSK1120212的组合对细胞生长抑制更敏感;然而,1μmol/L的GSK2118436和0.03μmol/L的Omipalisib也观察到了明显的活性。GSK2118436与GSK2126458联合使用的抗增殖作用不如GSK2118443和GSK1120212组合或GSK1120212和GSK2126485组合所观察到的有效。在YUSIT1 GSK2118436抗性克隆中观察到这些组合的益处,尽管GSK2126458与GSK2118443或GSK1120212的组合在这些克隆中具有适度的协同作用,几乎是相加的(补充表S1)。 |
| 体内研究 (In Vivo) |
在 BT474 人类肿瘤异种移植模型中,GSK2126458 治疗以剂量依赖性方式降低 pAkt-S473 水平,并在 300 μg/kg 的低剂量下以剂量依赖性方式抑制肿瘤生长。 GSK2126458在四种临床前物种(小鼠、大鼠、狗和猴)中的口服生物利用度也良好,但其血液清除率较低。 [1]
在体内环境中,Omipalisib/1在植入小鼠体内的人类BT474肿瘤中表现出pAKT-S473水平的剂量依赖性降低。在这项旨在测量药效学(PD)反应幅度和持续时间的研究中,小鼠口服药物,并在24小时内测定pAKT水平。单次服用300μg/kg剂量后,1在10小时的观察期内显示出深刻而持续的PD反应,pAKT水平在24小时后恢复到对照组水平(图4)。值得注意的是,持续的PD反应是在非常低的药物循环水平下实现的,与1的高体外效力一致。[1] 化合物1/Omipalisib也在BT474人肿瘤异种移植物生长疗效模型中进行了评估,其中小鼠每周口服五次,持续三周。与PI3K/AKT/mTOR通路的抑制一致,该药物表现出剂量依赖性的肿瘤生长抑制作用(图5)。在研究中,最高剂量(3 mg kg−1)耐受良好。如前所述,化合物2在BT474异种移植物中表现出疗效,每天两次,剂量为25mg kg-1。相比之下,化合物1在更低的剂量和更少的给药频率下表现出相似的疗效。 |
| 酶活实验 |
PI3K抑制的HTRF体外分析[1]
开发了PI3激酶谱测定法,用于测量PI3Kα、β、δ和γ异构体的化合物依赖性抑制作用,以及体外催化测定。该检测方法是由Upstate生产的试剂盒开发和优化的。简而言之,该程序利用四个结合伴侣之间预先形成的HTRF(均相时间分辨荧光能量转移)复合物:1)生物素化的PIP3,2)GST标记的pleckstrin同源(PH)结构域,3)铕标记的抗GST单克隆抗体,以及4)链霉抗藻蓝蛋白(APC)。PI 3-激酶活性产生的天然PIP3从PH结构域置换生物素-PIP3,导致HTRF复合物解离和荧光信号降低。该测定的格式对于PI3K的所有4种亚型都是相同的;差异在于用于实现最稳健信号的酶的浓度。α和δ测定在400pM酶下进行;β测定在200pM酶下进行,γ测定在1nM酶下进行。此外,α、β和δ测定用150mM NaCl进行,而γ测定在没有NaCl的情况下进行。在α、β和δ测定中,ATP浓度为100uM,在γ测定中为15uM ATP。所有反应均在10uM PIP2下进行。 使用 Multidrop Combi 将 2.5 μL 终止液(终止液 A 和终止液 B 分别以 5:1 的比例预混合)添加到所有孔中以猝灭反应。然后使用 Multidrop Combi 添加 2.5 μL 检测溶液(检测混合物 C、检测混合物 A 和检测混合物 B 以 18:1:1 的比例组合在一起,即,对于 6000 µL 总体积,混合 5400 µL 检测混合物 C、300 µL 检测混合物 A 和 300 µL 检测混合物 B)。请注意,该溶液需要在使用前两小时配制。暗孵育一小时后,在设置为 330nm 激发的 Envision 读板器上测量 HTRF 信号。 |
| 细胞实验 |
BT474、HCC1954 和 T-47D(人乳房)在含有 10% 胎牛血清的 RPMI-1640 中于 37°C、5% CO2 培养箱中培养。在进行测定设置之前,将细胞按一定密度分入 T75 烧瓶中,以便在测定收获时达到 70-80% 的汇合度。使用胰蛋白酶-EDTA 0.25% 收获细胞。利用台盼蓝排除染色,对细胞悬浮液进行细胞计数。然后,将细胞接种于 384 孔黑色平底聚苯乙烯培养皿中,每孔 1,000 个细胞,每培养皿 48 μL 培养基。第二天,所有板在 5% CO2、37 °C 下过夜后添加 GSK2126458。对一块板进行 CellTiter-Glo 处理,进行第 0 天 (t=0) 测量并按照以下说明进行读数。 GSK2126458 在 384 孔透明底部聚丙烯板中使用连续两倍稀释制备。将 4 μL 这些稀释液添加到 105 μL 培养基中并混合溶液后,将 2 μL 这些稀释液添加到细胞板的每个孔中。每孔中的最终 DMSO 浓度为 0.15%。细胞在 37°C 和 5% CO2 下孵育 72 小时。每个板在与 GSK2126458 一起孵育 72 小时后进行显色和读数。使用与孔中细胞培养物体积相等的体积,将 CellTiter-Glo 试剂添加到测定板中。在室温下孵育约 30 分钟并振荡两分钟后,使用 Analyst GT 读数器读取板的化学发光信号。结果针对 GSK2126458 的浓度绘制,并表示为 t=0 的百分比。对于 GSK2126458,通过使用 XLfit 软件将剂量响应与 4 或 6 参数曲线拟合来确定抑制 50% 细胞生长的浓度 (gIC50),其中 Y min 作为 t=0,Y max 作为 DMSO 对照。对于背景校正,从所有样品中减去不含细胞的孔的值。
|
| 动物实验 |
Human BT474 tumors implanted in mice.
≤300 μg /kg Administered via p.o. The pharmacokinetics of 1 (free base) were studied following single intravenous and/or oral administration to the male mouse, rat, dog and monkey. The IV and PO solution formulations contained 40% (v/v) PEG-400, 16% (w/v) encapsin in saline and water, respectively. The pH was adjusted to within 3.0-4.0 for the mouse, rat, dog and monkey solutions. Oral bioavailability was estimated using a cross-over study design for the dog and monkey (n = 3). Oral bioavailability in the rat was estimated using crossover (n = 1) and non-crossover (n = 2) designs and a non-crossover serial design was employed in the mouse (n = 2 IV and n = 3 PO). Blood samples were assayed for Omipalisib (GSK2126458, GSK458) using protein precipitation with acetonitrile followed by HPLC/MS/MS analysis employing positive-ion Turbo IonSpray ionization. Blood concentration-time data were analyzed by non-compartmental methods. Mouse and rat data reported as mean ± range. Dog and monkey data reported as mean ± standard deviation. [1] |
| 药代性质 (ADME/PK) |
The PK profile of 1/Omipalisib was studied in four preclinical species (mouse, rat, dog, and monkey). The compound showed low blood clearance and good oral bioavailability (Table 6). In addition, 1 had minimal potential to inhibit the human cytochrome P450 isoforms (IC50 > 25 μM vs CYPs 3A4, 1A2, 2C9, 2C19, and 2D6). [1]
Pharmacokinetic analyses for Omipalisib[3] Following single daily dosing, the median tmax ranged from 1 to 4 hours. Mean AUC0–24hrs and Cmax increased approximately in proportion with doses from 0.1 to 0.4 mg daily and then from 0.75 mg to 3.0 mg/day but not across the whole dose range (Supplementary Table S1 and Fig. 1A). As expected due to accumulation of OmipalisibGSK458, the mean AUC0–12hrs and Cmax were generally higher following the second dose versus the first dose with the twice-daily dosing schedule. The average time spent > 20 ng/mL (the target dose level based on preclinical data) was greater with twice-daily than once-daily dosing (21.2 hours at 2 mg twice daily vs. 14.5 hours at the once-daily MTD of 2.5 mg; Fig. 1B). The terminal T1/2 and AUC0–>∞ could not be determined due to the large %AUC extrapolation in >20% of patients. Because of between-subject variability, pharmacokinetic values overlapped across doses. The study results also highlight the importance of pharmacokinetic/pharmacodynamic data to define the optimal biologic dose in a first-in-patient study of a targeted anticancer therapy. Pharmacokinetic/pharmacodynamic modeling using mouse BT474 xenografts yielded a sustained mean target serum concentration of >20 ng/mL as the expected IC67 for AKT phosphorylation with range of 6.6 to 60 ng/mL to account for potential translation differences between mice and humans. Pharmacokinetic data from the daily dosing schedule suggested that the target serum concentration of >20 ng/mL was not being sustained over a 24-hour interval. In addition, significant interpatient variability in drug exposure was observed across the once-daily dosing levels. These two factors likely contributed to the lack of dose- and exposure-dependent effect observed in the pharmacodynamic analyses with daily dosing of Omipalisib/GSK458 and may have impacted the observed antitumor activity. The twice-daily dosing schedule achieved more consistent serum levels above the target exposure. Whether twice-daily dosing of GSK458 translates into more effective target inhibition and enhanced antitumor activity requires further clinical evaluation as pharmacodynamic analyses were nearly entirely limited to the once-daily dosing cohort and the MTD was not reached with twice-daily dosing.[3] GSK458/Omipalisib was fairly well tolerated. The frequency of adverse events appeared to be similar with once-daily versus twice-daily dosing. Diarrhea was a common clinical event; however, it was largely grade 1–2 in severity for most patients, with grade 3 diarrhea (>7 stools above baseline/day) observed in 8% of enrolled patients. Diarrhea appeared to be self-limiting and responsive to temporary dose interruptions and resolved in more than 80% of patients at the time of last study reporting. Hyperglycemia, a class effect (and potential pharmacodynamic biomarker) of PI3K pathway inhibition, was observed in 18% of patients on study and was mostly grade 1–2 in severity. Hyperglycemia was commonly managed with oral agents (e.g., metformin); the initiation of insulin during protocol therapy was a rare event. Other class effects of PI3K pathway inhibition were observed, including rash and mucositis, both managed effectively with temporary dose holds and/or initiation of topical steroid treatment. Interestingly, in contrast to other PI3K inhibitors such as BKM120, effects on mood were uncommon, suggesting potentially a differential penetration across the blood–brain barrier or other off-target differences in receptor inhibition.[3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Determination of MTD for Omipalisib
In the once-daily dose-escalation part of the study, 8 dose levels were explored (Table 3). The first DLT (grade 3 diarrhea) occurred at the 1.5 mg once-daily dose. This cohort was expanded without further dose limiting events. Three DLTs (all grade 3 diarrhea events) occurred at the 3 mg once-daily dose level, rendering this the nontolerated dose (NTD). Patients were subsequently treated at dose levels of 2 mg and 2.5 mg once daily without any observed DLTs, establishing 2.5 mg as the MTD with once daily dosing schedule.
Because of the observation of a shorter duration of Omipalisib/GSK458 drug levels above target range with daily dosing, twice-daily dose escalation was initiated at a dose of 0.75 mg twice daily, and 5 dose levels were studied (Table 3). No DLTs were observed at the 2 mg twice-daily dose level. One of the three patients experienced a DLT (grade 3 fatigue + grade 3 rash) at 2.5 mg twice daily; however, due to the decision to discontinue single-agent testing of GSK458, further patients were not treated at this dose level; and therefore, the MTD with twice-daily dosing could not be determined. Safety results for Omipalisib The most common adverse events (any grade severity) experienced on study were fatigue (45%), diarrhea (45%), nausea (42%), decreased appetite (30%), and vomiting (26%; Table 4). The most common grade ≥ 3 adverse events included diarrhea (8%), hyperglycemia (>250 mg/dL; 6%), and skin rash (5%). Nine patients (5%) experienced a treatment-related serious adverse event, including four patients with diarrhea. Diarrhea appeared to be an intermittent, self-limiting event for most patients, with resolution reported in 82% of patients. Rash was noted in 21 patients (12%), with most patients experiencing a single occurrence (81%). The most common type of rash was maculopapular in appearance; acneiform rash was rare (2 patients). Hyperglycemia was noted in 37 patients (22%), was mostly grade 1 or 2 in severity, and did not require dose adjustment in the majority of patients (92%). Cardiac toxicity was minimal with 2 (1%) patients experiencing post-baseline decreases in ejection fraction below the lower limit of normal and >10% from baseline. There were no significant effects on mood noted. There were no treatment-related grade 5 adverse events. |
| 参考文献 |
|
| 其他信息 |
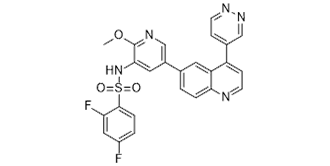
Omipalisib is a member of the class of quinolines that is quinoline which is substituted by pyridazin-4-yl and 5-[(2,4-difluorobenzene-1-sulfonyl)amino]-6-methoxypyridin-3-yl groups at positions 4 and 6, respectively. It is a highly potent inhibitor of PI3K and mTOR developed by GlaxoSmithKline and was previously in human phase 1 clinical trials for the treatment of idiopathic pulmonary fibrosis and solid tumors. It has a role as an autophagy inducer, an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor, a mTOR inhibitor, an antineoplastic agent, a radiosensitizing agent and an anticoronaviral agent. It is a member of quinolines, a difluorobenzene, a sulfonamide, an aromatic ether, a member of pyridines and a member of pyridazines.
Omipalisib has been used in trials studying the treatment of CANCER, Solid Tumours, and Idiopathic Pulmonary Fibrosis. Omipalisib is a small-molecule pyridylsulfonamide inhibitor of phosphatidylinositol 3-kinase (PI3K) with potential antineoplastic activity. Omipalisib binds to and inhibits PI3K in the PI3K/mTOR signaling pathway, which may trigger the translocation of cytosolic Bax to the mitochondrial outer membrane, increasing mitochondrial membrane permeability and inducing apoptotic cell death. Bax is a member of the proapoptotic Bcl2 family of proteins. PI3K, often overexpressed in cancer cells, plays a crucial role in tumor cell regulation and survival. Phosphoinositide 3-kinase α (PI3Kα) is a critical regulator of cell growth and transformation, and its signaling pathway is the most commonly mutated pathway in human cancers. The mammalian target of rapamycin (mTOR), a class IV PI3K protein kinase, is also a central regulator of cell growth, and mTOR inhibitors are believed to augment the antiproliferative efficacy of PI3K/AKT pathway inhibition. 2,4-Difluoro-N-{2-(methyloxy)-5-[4-(4-pyridazinyl)-6-quinolinyl]-3-pyridinyl}benzenesulfonamide (Omipalisib/GSK2126458, 1) has been identified as a highly potent, orally bioavailable inhibitor of PI3Kα and mTOR with in vivo activity in both pharmacodynamic and tumor growth efficacy models. Compound 1 is currently being evaluated in human clinical trials for the treatment of cancer.[1] In conclusion, we report the discovery of 1/Omipalisib, a structurally novel inhibitor of the PI3K/AKT/mTOR signaling pathway with picomolar activity against PI3Kα and mTOR. Compound 1 displays remarkable potency in both mechanistic and antiproliferative cellular assays. Compound 1 also exhibits excellent in vivo activity, highlighted by a sustained PD effect at very low circulating drug levels. Inhibition of the PI3K/AKT/mTOR pathway is expected to have a beneficial effect on cancer therapy, and 1 has been advanced into a phase I, open-label, dose-escalation study in subjects with solid tumors or lymphoma.[1] Recent results from clinical trials with the BRAF inhibitors GSK2118436 (dabrafenib) and PLX4032 (vemurafenib) have shown encouraging response rates; however, the duration of response has been limited. To identify determinants of acquired resistance to GSK2118436 and strategies to overcome the resistance, we isolated GSK2118436 drug-resistant clones from the A375 BRAF(V600E) and the YUSIT1 BRAF(V600K) melanoma cell lines. These clones also showed reduced sensitivity to the allosteric mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitor GSK1120212 (trametinib). Genetic characterization of these clones identified an in-frame deletion in MEK1 (MEK1(K59del)) or NRAS mutation (NRAS(Q61K) and/or NRAS(A146T)) with and without MEK1(P387S) in the BRAF(V600E) background and NRAS(Q61K) in the BRAF(V600K) background. Stable knockdown of NRAS with short hairpin RNA partially restored GSK2118436 sensitivity in mutant NRAS clones, whereas expression of NRAS(Q61K) or NRAS(A146T) in the A375 parental cells decreased sensitivity to GSK2118436. Similarly, expression of MEK1(K59del), but not MEK1(P387S), decreased sensitivity of A375 cells to GSK2118436. The combination of GSK2118436 and GSK1120212 effectively inhibited cell growth, decreased ERK phosphorylation, decreased cyclin D1 protein, and increased p27(kip1) protein in the resistant clones. Moreover, the combination of GSK2118436 or GSK1120212 with the phosphoinositide 3-kinase/mTOR inhibitor Omipalisib/GSK2126458 enhanced cell growth inhibition and decreased S6 ribosomal protein phosphorylation in these clones. Our results show that NRAS and/or MEK mutations contribute to BRAF inhibitor resistance in vitro, and the combination of GSK2118436 and GSK1120212 overcomes this resistance. In addition, these resistant clones respond to the combination of GSK2126458 with GSK2118436 or GSK1120212. Clinical trials are ongoing or planned to test these combinations.[2] Purpose: Omipalisib/GSK2126458 (GSK458) is a potent inhibitor of PI3K (α, β, γ, and δ), with preclinical studies demonstrating broad antitumor activity. We performed a first-in-human phase I study in patients with advanced solid tumors. Materials and methods: Patients received oral GSK458 once or twice daily in a dose-escalation design to define the maximum tolerated dose (MTD). Expansion cohorts evaluated pharmacodynamics, pharmacokinetics, and clinical activity in histologically and molecularly defined cohorts. Results: One hundred and seventy patients received doses ranging from 0.1 to 3 mg once or twice daily. Dose-limiting toxicities (grade 3 diarrhea,n= 4; fatigue and rash,n= 1) occurred in 5 patients (n= 3 at 3 mg/day). The MTD was 2.5 mg/day (MTD with twice daily dosing undefined). The most common grade ≥3 treatment-related adverse events included diarrhea (8%) and skin rash (5%). Pharmacokinetic analyses demonstrated increased duration of drug exposure above target level with twice daily dosing. Fasting insulin and glucose levels increased with dose and exposure of Omipalisib/GSK458. Durable objective responses (ORs) were observed across multiple tumor types (sarcoma, kidney, breast, endometrial, oropharyngeal, and bladder cancer). Responses were not associated withPIK3CAmutations (OR rate: 5% wild-type vs. 6% mutant). Conclusions: Although the MTD of GSK458 was 2.5 mg once daily, twice-daily dosing may increase duration of target inhibition. Fasting insulin and glucose levels served as pharmacodynamic markers of drug exposure. Select patients achieved durable responses; however,PIK3CAmutations were neither necessary nor predictive of response. Combination treatment strategies and novel biomarkers may be needed to optimally target PI3K.[3] |
| 分子式 |
C25H17F2N5O3S
|
|---|---|
| 分子量 |
505.4960
|
| 精确质量 |
505.102
|
| 元素分析 |
C, 59.40; H, 3.39; F, 7.52; N, 13.85; O, 9.50; S, 6.34
|
| CAS号 |
1086062-66-9
|
| 相关CAS号 |
1086062-66-9
|
| PubChem CID |
25167777
|
| 外观&性状 |
light yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
715.6±70.0 °C at 760 mmHg
|
| 熔点 |
187-189℃
|
| 闪点 |
386.6±35.7 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.660
|
| LogP |
3.81
|
| tPSA |
115.34
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
833
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C1C([H])=C([H])C(=C([H])C=1F)F)(N([H])C1=C(N=C([H])C(=C1[H])C1C([H])=C([H])C2C(=C(C([H])=C([H])N=2)C2=C([H])N=NC([H])=C2[H])C=1[H])OC([H])([H])[H])(=O)=O
|
| InChi Key |
CGBJSGAELGCMKE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3
|
| 化学名 |
2,4-difluoro-N-[2-methoxy-5-(4-pyridazin-4-ylquinolin-6-yl)pyridin-3-yl]benzenesulfonamide
|
| 别名 |
Omipalisib; GSK2126458; GSK 2126458; 2,4-difluoro-N-(2-methoxy-5-(4-(pyridazin-4-yl)quinolin-6-yl)pyridin-3-yl)benzenesulfonamide; 2,4-difluoro-N-[2-methoxy-5-(4-pyridazin-4-ylquinolin-6-yl)pyridin-3-yl]benzenesulfonamide; GSK-212; GSK-2126458
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~197.8 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.95 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 1%DMSO+30% polyethylene glycol+1%Tween 80: 18mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9782 mL | 9.8912 mL | 19.7824 mL | |
| 5 mM | 0.3956 mL | 1.9782 mL | 3.9565 mL | |
| 10 mM | 0.1978 mL | 0.9891 mL | 1.9782 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00972686 | Completed | Drug: GSK2126458 | Solid Tumor | GlaxoSmithKline | August 31, 2009 | Phase 1 |
| NCT01725139 | Completed | Drug: Placebo Drug: GSK2126458 |
Idiopathic Pulmonary Fibrosis | GlaxoSmithKline | March 8, 2013 | Phase 1 |
Greger JG, et al. Mol Cancer Ther. 2012, 11(4), 909-920. |
Developing selective type II kinase inhibitors. (A) Docking imatinib into the X-ray co-crystal structure of DDR1. (B) Chemical structure of DDR1-IN-1/2 and representative developing rationale.ACS Chem Biol.2013 Oct 18;8(10):2145-50. |
Combinatorial Screening of DDR1-IN-1/2 with the LINCS library against the SNU-1040 cell line.ACS Chem Biol.2013 Oct 18;8(10):2145-50. |