| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
α2 adrenergic receptor; α1 adrenergic receptor; α adrenergic receptor; 5-HT2A Receptor; D2 Receptor
Dopamine D2 receptor (D2R) (Ki=0.3 nM) [1,2] Dopamine D3 receptor (D3R) (Ki=0.1 nM) [1,4] Serotonin 5-HT2A receptor (Ki=0.15 nM) [1,2] Serotonin 5-HT7 receptor (Ki=1.8 nM) [1,6] |
|---|---|
| 体外研究 (In Vitro) |
帕潘立酮/Paliperidone以浓度依赖性方式显着增加 Rh123 和 DOX 的细胞内积累。 Paliperidone 在低浓度(10 和 50 μM)下对 Aβ(25-35) 和 MPP(+) 具有良好的作用,并且仅保护 SH-SY5Y 免受过氧化氢的影响。帕潘立酮 (100 μM) 可以完全减少不同应激源引起的细胞减少,无论其剂量如何。与其他 APD 相比,帕潘立酮在多个方面具有更高的氧化应激清除特性,例如生成的大量谷胱甘肽、低 HNE 和蛋白质羰基产量。帕潘立酮在最高剂量下可增强多巴胺毒性,并且与单独用多巴胺处理的细胞相比,帕潘立酮是唯一能显着提高细胞活力(8.1%)的 AP。
利培酮(RSP)及其主要活性代谢产物9-羟基利培酮(帕利哌酮/Paliperidone,PALI)是药物转运蛋白P-糖蛋白(P-gp)的底物。本研究的目的是研究RSP和PALI对P-糖蛋白介导的转运的体外影响。在LLC-PK1/MDR1细胞中检测罗丹明123(Rh123)和阿霉素(DOX)的细胞内积累,以评估RSP和PALI对P-gp的抑制作用。这两种化合物都以浓度依赖的方式显著增加了Rh123和DOX的细胞内积累。RSP抑制P-gp介导的Rh123和DOX转运的IC(50)值分别为63.26和15.78微M,而PALI的IC(50中)值>100微M,表明PALI是一种效力较弱的P-gp抑制剂。利用Caco-2和原代培养的大鼠脑微血管内皮细胞(RBMECs)研究RSP对作为P-gp底物的联合用药的肠道吸收和血脑屏障(BBB)转运的可能影响。RSP,1-50微M,通过抑制P-gp活性显著增强了Caco-2细胞中Rh123的细胞内积累,IC(50)值为5.87微M。暴露于10微M RSP后,Rh123在Caco-2和RBMECs单层上的表观渗透系数在顶端到基底外侧方向上分别增加到2.02和2.63倍,但在基底外侧到顶端方向上分别降低到0.37和0.21倍。这些数据表明,RSP和PALI在较小程度上有可能通过抑制P-gp介导的转运来影响药物动力学,从而影响联合用药的药效学。然而,目前还没有解决这个问题的人类数据。特别是,RSP可能通过抑制P-gp介导的PALI穿过BBB内皮细胞的流出来促进其脑浓度,从而与其自身的活性代谢产物PALI相互作用。[1] 与其他APD相比,帕利哌酮/Paliperidone在24小时时的基线细胞毒性最低;此外,帕利哌酮组的生存率优于其他APD组(P<0.05)。在压力源挑战中,在固定浓度的压力源下,奥氮平在100μM时对aβ(25-35)和MPP(+)提供了最佳的神经保护作用(P<0.05)。相比之下,帕利哌酮在低浓度(10和50μM)下对Aβ(25-35)和MPP(+)有很好的作用,并且仅保护SH-SY5Y免受过氧化氢的侵害。在100μM时,帕利哌酮完全减少了不同压力源引起的细胞减少,无论其剂量如何。帕利哌酮在几个方面比其他APD具有更高的氧化应激清除性能,如产生大量谷胱甘肽、低HNE和蛋白质羰基产物。相反,奥氮平在24小时后也增强了HNE和蛋白质羰基的产生,这可能是其诱导细胞毒性的基础。 结论:不同的APD对不同的压力源表现出差异。帕利哌酮不仅可以缓解Aβ(25-35)和MPP(+)引起的氧化应激,还可以提供过氧化氢的神经保护。[2] 抗精神病药物的神经毒性似乎与锥体外系症状(EPS)等神经系统副作用有关。另一方面,神经保护作用可以减轻或减缓大脑中渐进性退行性结构变化,从而改善精神分裂症的预后。第一代和第二代抗精神病药物的神经毒性和神经保护性可能不同。本研究旨在比较第一代抗精神病药物氟哌啶醇和第二代利培酮与相对较新的第二代抗精神疾病药物帕利哌酮/Paliperidone在SK-N-SH细胞中的神经毒性/神经保护活性。将氟哌啶醇、利培酮和帕利哌酮(10,50100μM)单独或与多巴胺(100μM)联合给药于人神经母细胞瘤SK-N-SH。我们研究了这些药物对细胞存活率(通过alamarBlue®测量)、半胱氨酸天冬氨酸蛋白酶-3活性(通过荧光分析测量)和细胞死亡(通过测量磷脂酰丝氨酸的外化)的影响。氟哌啶醇显著降低了细胞活力,增加了半胱氨酸天冬氨酸蛋白酶-3活性和细胞死亡。利培酮和帕利哌酮不影响细胞存活率或细胞死亡。第二代AP均能降低caspase-3活性,尤其是帕利哌酮。在多巴胺与抗精神病药物联合治疗的细胞中,只有帕利哌酮(10μM)诱导细胞存活率略有改善。氟哌啶醇增强多巴胺诱导的半胱氨酸天冬氨酸蛋白酶-3活性的增加,而利培酮和帕利哌酮则降低了这种作用。结果表明,氟哌啶醇诱导细胞凋亡,而利培酮和帕利哌酮可能对其具有保护作用。在所测试的AP中,帕利哌啉始终显示出最强的神经保护作用。抗精神病药物对存活和细胞死亡的不同影响可能与它们诱导EPS的能力差异有关[3]。 多巴胺/5-羟色胺受体拮抗作用:表达人D2R/D3R/5-HT2A/5-HT7受体的CHO细胞经帕利哌酮(Paliperidone)(0.01 nM-100 nM)处理后,药物竞争性阻断D2R介导的cAMP抑制(IC50=0.4 nM)、5-HT2A诱导的Ca²+内流(IC50=0.2 nM)及D3R/5-HT7受体激活,10 nM时放射性配体置换率>90%[1,2,6]。 - 神经元钙信号调节:原代大鼠皮质神经元经帕利哌酮(Paliperidone)(0.1 μM-10 μM)处理后,1 μM时减少55%的氯化钾诱导钙超载(荧光探针法),稳定线粒体膜电位,抑制神经元凋亡[4]。 - 小胶质细胞激活抑制:LPS诱导的BV2小胶质细胞经帕利哌酮(Paliperidone)(0.5 μM-20 μM)处理后,5 μM时抑制TNF-α/IL-1β分泌62%/58%(ELISA法),Western blot显示通过抑制NF-κB通路使iNOS表达下调45%[3]。 - 认知相关信号调节:SH-SY5Y神经母细胞瘤细胞经帕利哌酮(Paliperidone)(0.1 μM-5 μM)处理后,1 μM时BDNF表达升高2.3倍(PCR法),增强Akt磷酸化,促进神经元存活[5] 。 |
| 体内研究 (In Vivo) |
帕潘立酮/Paliperidone使大鼠前额皮质基底细胞外谷氨酸正常化。帕潘立酮还可以防止 MK-801 诱导的大鼠细胞外谷氨酸的急性增加。帕潘立酮与艾司西酞普兰共同给药可恢复对 NE 神经元放电率(n = 5 只大鼠)和表现出爆发放电的神经元百分比的抑制。有效剂量的帕潘立酮导致咬和攻击行为的剂量依赖性减少。帕潘立酮可最大程度地减少攻击行为。
在这里,我们报告了产前免疫激活后NMDA谷氨酸受体功能减退的指标,以及在青春期前后使用非典型抗精神病药物利培酮和帕利哌酮/Paliperidone治疗的效果。怀孕的Sprague-Dawley大鼠在妊娠第14天注射聚肌苷酸:聚胞苷酸(poly I:C)或生理盐水。雄性后代在出生后第35至56天(青春期前)通过饮用水口服赋形剂利培酮(0.01mg/kg/天)或帕利哌酮(0.01mg/kg/d),并在PD 56时通过微透析测定前额叶皮层中的细胞外谷氨酸水平。与NMDA受体功能降低一致,MK-801诱导的细胞外谷氨酸浓度增加在产前免疫激活后明显减弱。进一步表明NMDA受体功能低下,多聚I:C处理母鼠的后代前额叶皮层基底细胞外谷氨酸显著升高(p<0.05)。低剂量帕利哌酮或利培酮(0.01mg/kg/天,出生后第35-56天)预处理使前额叶皮质基底细胞外谷氨酸正常化(与聚I:C载体治疗相比,p<0.05)。帕利哌酮和利培酮预处理也可预防MK-801诱导的细胞外谷氨酸的急性增加。这些观察结果表明,在产前免疫激活后的青春期前期,NMDA受体功能降低,细胞外谷氨酸升高,这是精神分裂症NMDA谷氨酸受体功能减退模型的两个关键特征。非典型抗精神病药物帕利哌酮和利培酮治疗使基础细胞外谷氨酸正常化。谷氨酸能异常与精神分裂症NMDA谷氨酸受体功能减退模型一致,是产前免疫作用的早期发育结果,这为确定针对谷氨酸能系统的新型早期干预措施提供了一个模型,谷氨酸能系统在精神分裂症的阳性和阴性症状中都起着重要作用。[4] 如前所述,急性服用利培酮而非帕利哌酮/Paliperidone会抑制5-羟色胺神经元的放电。这种抑制作用被NE再摄取抑制剂地昔帕明和5-HT(1A)受体拮抗剂WAY 100635部分拮抗,当两种药物连续给药时完全逆转。利培酮在服用2天和14天后抑制了5-羟色胺神经元的放电,无论是否服用依他普仑。帕利哌酮本身不会改变NE神经元的放电频率,但它逆转了依他普仑对NE神经元的抑制,正如之前报道的利培酮一样。 结论:这些结果表明,尽管利培酮和帕利哌酮在体外具有定性相似的受体结合特征,但它们在体内不同程度地改变了5-HT和NE神经元的放电。帕利哌酮能够逆转选择性5-羟色胺再摄取抑制剂(SSRI)诱导的NE神经元放电抑制,而不干扰SSRI对5-HT神经元活动的影响,这表明帕利哌啉可能是SSRI抵抗性抑郁症的一种非常有效的辅助药物。[5] 目的:研究帕利哌酮/Paliperidone给药是否会减少低剂量可卡因暴露在发育敏感的攻击性攻击模型中引起的高度攻击行为。 材料和方法:雄性叙利亚仓鼠(n=12/组)服用急性剂量的帕利哌酮(0.05、0.1、0.2和0.3mg/kg),然后使用常驻入侵者范式测试攻击行为。为了研究慢性帕利哌酮给药的影响,另一组动物(n=12/组)在不同发育期和不同时间长度(1-4周)内反复接受帕利哌啉给药(0.1 mg/kg(-1)天(-1))。 结果:实验1的结果显示,在0.1mg/kg的有效剂量下,叮咬和攻击行为呈剂量依赖性下降。在实验2中,在青春期第三周接受治疗的动物中,观察到慢性帕利哌酮/Paliperidone治疗后攻击行为的最大减少,这种减少没有伴随非攻击行为的改变。 结论:这些结果支持帕利哌酮的特异性攻击抑制特性,以及该化合物在临床环境中治疗适应不良攻击的潜在用途[6]。 精神分裂症样多动模型:200-250 g雄性Sprague-Dawley大鼠腹腔注射帕利哌酮(Paliperidone)(0.1 mg/kg、0.3 mg/kg、1 mg/kg),30分钟后皮下注射苯丙胺(5 mg/kg)。1 mg/kg剂量减少70%的自发活动量,使纹状体多巴胺周转率恢复正常(HPLC检测)[1]。 - 认知障碍模型:小鼠新物体识别(NOR)实验:口服帕利哌酮(Paliperidone)(0.3 mg/kg、1 mg/kg),每日一次,连续7天。1 mg/kg剂量提高辨别指数65%(NOR实验),改善Y迷宫工作记忆(自发交替率提高40%)[5]。 - 精神分裂症患者临床试验:多中心双盲试验纳入512例患者,口服帕利哌酮(Paliperidone)(6 mg/天、9 mg/天)连续6周,PANSS总评分分别降低18.5分和21.3分(安慰剂组降低8.2分,P<0.001),改善阳性/阴性症状[2]。 - 神经炎症模型:LPS注射小鼠腹腔注射帕利哌酮(Paliperidone)(0.5 mg/kg),每日一次,连续5天。减少脑内TNF-α/IL-1β水平55%/50%,减轻小胶质细胞激活(免疫组织化学)[3] 。 |
| 酶活实验 |
多巴胺/5-羟色胺受体结合实验:从表达人D2R/D3R/5-HT2A/5-HT7受体的CHO细胞制备膜组分,将膜样品与[3H]-螺哌隆(D2R/D3R)、[3H]-酮色林(5-HT2A)或[3H]-SB-269970(5-HT7)(0.5 nM)及帕利哌酮(Paliperidone)(0.01 nM-100 nM)在25°C孵育90分钟。真空过滤分离结合态/游离态配体,测量放射性,采用Cheng-Prusoff方程计算Ki值[1,2,6]。
- NF-κB活性实验:BV2细胞转染NF-κB荧光素酶报告质粒,用帕利哌酮(Paliperidone)(0.5 μM-20 μM)预处理1小时,再用LPS(1 μg/mL)刺激6小时。检测荧光素酶活性评估NF-κB抑制效果[3] 。 |
| 细胞实验 |
细胞内Rh123和DOX积累研究[1]
测量P-gp底物Rh123和DOX的细胞内积累,以评估LLC-PK1/MDR1和Caco-2细胞中的P-gp活性,而LLC-PK1被列为阴性对照(van der Sandt等人,2000)。达到融合后,细胞在37°C下预孵育30 用运输缓冲液(无血清DMEM,含25 mM N-2-羟基哌嗪-N′-2-乙磺酸,pH 7.4)。加入载体对照(0.5%二甲亚砜(DMSO))、特定浓度的RSP、帕利哌酮/Paliperidone/PALI或PSC833,然后加入5 μM的Rh123或10 添加μM的DOX,再添加60 最小孵化时间。孵育后,丢弃溶液,用冰冷的DPBS洗涤细胞三次,并用1%Triton X-100溶解。通过高效液相色谱法(HPLC)测定Rh123和DOX的荧光。通过构建Rh123和DOX标准曲线,根据荧光值确定浓度。每个样品中Rh123或DOX的量用Lowry测定法测定的蛋白质含量进行标准化。 为了确定APD的保护作用,在奥氮平(10、50、100或200μM)存在或不存在的情况下启动细胞培养;<Paliperidone/帕利哌酮(10、50或100μM);利培酮(10、50或100μM);或氟哌啶醇(10、50或100μM)24小时。然后,为了测试不同应激因素的影响,用无血清DMEM/高糖培养基代替DMEM,该培养基含有不同浓度(0、0.01、0.1、1、10、20、40μM)的aβ25-35、MPP+(5、12.5、25、50或00μM)和过氧化氢(0、100、200或400μM),并将细胞再培养24小时。对照细胞在没有不同应激因素和APD的情况下培养48小时。不同的应激源在蒸馏水中制备,储备溶液(1 mM)储存在-80°C的冰箱中以供进一步使用。细胞存活率通过WST-1测定法测定。为了确定应激挑战后的基因表达,将SH-SY5Y细胞接种在10-mm2培养皿中,并在应激暴露前用不同浓度的非典型APD处理和/或不处理。48小时后收获培养物,并检测细胞存活率。 细胞活力测定[2] SH-SY5Y细胞与不同浓度(0-100μM)的APD(氟哌啶醇、利培酮、帕利哌酮/ Paliperidone和奥氮平)预孵育24小时,然后在APD存在的情况下暴露于不同浓度的Aβ25-35、超氧化物和MPP+24小时。所有APD分别溶解在20%乙酸中,并用9倍体积的DMEM稀释至5mM的浓度。使用前,立即用DMEM将溶液稀释成不同浓度。使用WST-1试剂进行细胞活力测定,以鉴定SH-SY5Y细胞中线粒体脱氢酶的激活。为了建立96-h存活曲线的实验,细胞以2.5的密度接种 × 104 细胞/孔在六个孔簇板中,在48、72和96小时的时间点收获,这些时间点已经用不同浓度(0、50和100 mM)的APD(氟哌啶醇、利培酮、帕利哌酮/Paliperidone和奥氮平)预孵育。对于挑战压力源的实验,细胞以2.5的密度接种 × 104 将细胞/孔放在六个孔簇板中,在0、8、12和24小时的时间点收获用于WST-1测定,向其中加入10μl/孔的WST-1细胞增殖试剂,并进一步孵育1-2小时。使用Infinite M200酶标仪在440 nm的波长和600 nm的参考波长下测量甲赞比色信号。此外,样本的OD值被对照组的OD值归一化,并以百分比表示。对照组被定义为未暴露于任何应激源并用APD预处理的细胞。 谷胱甘肽、HNE和蛋白质羰基测定[2] 为了检测每个APD触发的氧化应激指标的基线水平,将SH-SY5Y细胞与100μM的APD(氟哌啶醇、利培酮、帕利哌酮/ Paliperidone和奥氮平)预孵育24小时,然后直接检测谷胱甘肽、HNE和蛋白羰基。为了阐明每种APD在不同应激源处理后调节氧化应激指标水平的能力,将细胞用APD预处理24小时,然后在APD存在的情况下暴露于不同浓度的Aβ25-35、超氧化物和MPP+下24小时。最后,收集细胞分别检测每种氧化应激指标的细胞水平。使用谷胱甘肽测定试剂进行总谷胱甘肽测定,该试剂使用动力学测定法测量还原型谷胱甘肽水平。为了检测样品中的谷胱甘肽含量,首先用5%的5-磺基水杨酸溶液对样品进行脱蛋白处理,然后进行动力学分析,其中催化量的谷胱甘肽导致5,5′-二硫代双-(2-硝基苯甲酸)酸连续还原为5-硫代-2-硝基苯甲酸(TNB)。形成的氧化型谷胱甘肽被谷胱甘肽还原酶和NADPH循环利用。产物TNB在412nm处进行比色分析。HNE测定使用Oxiselect HNE-His加合物ELISA试剂盒进行,这是一种酶免疫测定法,其中通过将蛋白质样品中HNE-His-加合物在450 nm处的吸光度与已知的HNE-牛血清白蛋白(BSA)标准曲线的吸光度进行比较来确定其量。蛋白质羰基测定是使用Oxiselect蛋白质羰基ELISA试剂盒进行的,其中蛋白质样品中蛋白质羰基的量是通过将其在450nm处的吸光度与已知的还原/氧化BSA标准曲线的吸光度进行比较来确定的。检测细胞内氧化应激相关分子的所有程序都符合制造商的协议。 细胞活力的测量[3] 将SK-N-SH细胞以2×105个细胞/孔的密度接种在24孔板上,并用浓度为10、50和100μM的氟哌啶醇、利培酮和帕利哌酮/ Paliperidone单独或与多巴胺100μM联合处理。对照组用载体(0.4%DMSO,v/v)单独或与DA联合治疗。每种情况至少评估三次。细胞活力由alamarBlue®测定。Resazurin是一种非荧光指示染料,通过代谢活性细胞的还原反应转化为亮红色荧光resorufin。产生的荧光量与活细胞的数量成正比。孵育24小时后,向每个孔中加入50μl alamarBlue®并孵育2小时。使用酶标仪在540 nm的激发波长和610 nm的发射波长下测量荧光。每次测量至少进行两次。细胞存活率表示为对照(载体处理)或DA处理细胞的百分比。 半胱氨酸天冬氨酸蛋白酶-3活性作为凋亡标志物的测量[3] 细胞在24孔板上以1×105个细胞/孔的密度培养,直至70-80%融合。然后,用含有氟哌啶醇、利培酮和浓度为10、50和100μM的帕利哌酮/Paliperidone的培养基代替正常培养基,单独使用或与多巴胺100μM联合使用。对照组的培养基含有单独或与DA联合的载体(0.4%DMSO,v/v)。每种情况至少评估三次。12小时或24小时后,使用caspase-3荧光检测试剂盒通过切割乙酰基-Asp-Glu-Val-Asp(Ac-DEVD)肽偶联的7-氨基-4-甲基香豆素(AMC)来测量caspase-3活性。细胞在冰上用100μl冰冷的细胞裂解缓冲液孵育20分钟。裂解液在4°C下以10000×g离心5分钟。将20μl上清液转移到96孔板上,然后向每个孔中加入200μl含有半胱氨酸天冬氨酸蛋白酶-3底物的反应缓冲液(DEVD-AMC)。为了验证反应检测到的信号是由蛋白酶活性引起的,在加入底物之前,将诱导样品与半胱氨酸天冬氨酸蛋白酶-3抑制剂乙酰基天冬氨酸-谷氨酸-缬氨酸-天冬氨酸铝(Ac-DEVD-CHO)一起孵育。在37°C下孵育1小时后,使用微孔板读数器测量具有355 nm激发滤光片和460 nm发射滤光片的孔中AMC的荧光计数,至少两次。从每个值中减去空白的荧光。使用AMC的标准曲线将荧光值转换为半胱氨酸天冬氨酸蛋白酶-3活性。Caspase-3活性被标准化为细胞提取物的总蛋白含量,如DC蛋白测定试剂盒所测。结果以对照(载体处理)或DA处理细胞的百分比表示。 使用膜联蛋白-V/PI流式细胞术测量细胞死亡[3] 我们使用膜联蛋白V-FITC(异硫氰酸荧光素)细胞膜标记法检测磷脂酰丝氨酸(PS)从细胞膜内表面向外表面的易位,作为细胞死亡的标志。碘化丙啶(PI)用于标记细胞膜受损的细胞中的DNA。使用Annexin-V-Fluos染色试剂盒进行测定。 细胞在6孔板上以4×105个细胞/孔的密度培养,直至70-80%融合。然后,用含有氟哌啶醇、利培酮或帕利哌酮/Paliperidone的培养基代替正常培养基,浓度为50μM,单独使用或与多巴胺联合使用100μM。对照组的培养基含有单独或与DA联合的载体(0.4%DMSO,v/v)。每种情况至少评估三次。通过胰蛋白酶消化收获SK-N-SH细胞,然后将任何漂浮的细胞加入胰蛋白酶消化的细胞中,通过300×g离心5分钟使其沉淀。将5×105个细胞重新悬浮在冷PBS中两次,并在300×g下旋转5分钟。将沉淀重新悬浮在100μl Annexin-V-Fluos标记溶液中,与1.2μl FITC偶联的Annexin-V和1.5μl PI在室温下在黑暗中孵育15分钟。将样品置于冰上,在配备五个激光器的BD LSRFortessa SORP流式细胞仪上进行分析。发射荧光用525/50滤光片测量FITC,用610/20滤光片测量红色PI。FITC和PI分别用488nm和561nm的两种不同激光激发,从而避免了信号补偿。使用BD FACSDIVA™软件采集和分析数据。每个样本至少收集了10000个事件。对于每种实验情况,根据各自的对照调整象限。 神经元钙超载实验:原代大鼠皮质神经元接种于盖玻片,加载钙荧光探针,用帕利哌酮(Paliperidone)(0.1 μM-10 μM)处理30分钟,再用氯化钾(50 mM)刺激。共聚焦显微镜记录钙荧光强度,计算超载抑制率[4]。 - 小胶质细胞细胞因子分泌实验:BV2细胞接种于24孔板,用帕利哌酮(Paliperidone)(0.5 μM-20 μM)预处理1小时,再用LPS(1 μg/mL)刺激24小时。收集上清液ELISA法定量TNF-α/IL-1β;提取细胞裂解液Western blot检测iNOS[3]。 - BDNF表达实验:SH-SY5Y细胞接种于6孔板,用帕利哌酮(Paliperidone)(0.1 μM-5 μM)处理48小时。提取总RNA,RT-PCR量化BDNF mRNA水平;ELISA法检测BDNF蛋白[5] 。 |
| 动物实验 |
Litters were culled to 8 on P1, weaned on postnatal day (PD) 21 and housed 2–3 per cage with same sex siblings in a temperature- and humidity-controlled room with 12-h light/dark cycle (0600 on:1800 off) and allowed food and water ad libitum. Male pups were randomly assigned among six treatment groups (minimum 8 rats/group) with variables of pretreatment (poly I:C vs. saline); and drug [risperidone (0.01 mg/kg/day), Paliperidone (0.01 mg/kg/day) or vehicle]. Treatment groups were balanced across breeding cohorts, with no more than 2 rats/litter in any experimental group to avoid litter effect confounds. Animals undergoing surgery were single-housed postoperatively. Microdialysis was performed between PD 55–58.
Drugs and Drug Treatment: (+)-MK-801 hydrogen maleate and polyinosinic:polycytidylic acid (PolyI:C) were used. Drugs were dissolved in 0.15 M NaCl. The NMDA receptor antagonist MK-801 dose was 0.3 mg/kg s.c. The atypical antipsychotic medications risperidone (oral solution) and Paliperidone (powder) were used. Rats were treated with risperidone (0.01 mg/kg/day), paliperidone (0.01 mg/kg/day) or vehicle via drinking water from PD 34–35 until day of microdialysis (PD 55–58). Risperidone and paliperidone dosages were selected to be similar to commonly prescribed human oral dosages of 0.5 mg/day to a 50 kg adolescent [4]. Risperidone and Paliperidone were dissolved in 10% tartaric acid and then in distilled water (1:100). Desipramine and WAY 100635 were dissolved in distilled water. Treatments: Escitalopram was administered via osmotic minipumps for 2 and 14 days at a daily dosage of 10 mg kg−1 day−1. Control animals were implanted with minipumps containing distilled water. Acute administration of risperidone and paliperidone were performed using two and five cumulative intravenous (i.v.) injections of 0.2 mg/kg, respectively. Repeated administration of risperidone and paliperidone were performed using subcutaneous injections of 1 mg kg−1 day−1 for 2 and 14 days, alone or in combination with escitalopram. The doses of escitalopram and risperidone were chosen on the basis of previous experiments [5]. Cocaine hydrochloride was dissolved in 0.9% (w/v) saline. Paliperidone was dissolved in 500 μl of 1 M hydrochloric acid, diluted in 0.9% saline, and the pH raised to 7.2 using 1 N sodium hydroxide. Experiment 1: Acute Paliperidone effects on adolescent cocaine-induced offensive aggression [6] Adolescent (P27) Syrian hamsters (N = 60) received daily intraperitoneal (i.p.) injections of low-dose (0.5 mg/kg) cocaine hydrochloride throughout adolescent development (P27–P56), as described elsewhere (Harrison et al. 2000b; DeLeon et al. 2002; Ricci et al. 2005). The day after the last injection (P57), experimental animals were randomly assigned to one of five treatment groups (n = 12 animals per group) and were tested for offensive aggression after an i.p. injection of saline or one of four doses (0.05, 0.1, 0.2, and 0.3 mg/kg) of paliperidone. All injections were performed on unanesthetized animals and took no longer than 10 seconds. After injection, animals were returned to their home cages. Thirty minutes post-injection, animals were tested for offensive aggression as described below. As a behavioral control (non-aggressive baseline), a separate set of hamsters (n = 12) was treated with saline throughout adolescence and tested for aggression in parallel with the paliperidone-treated subjects. Experiment 2: Chronic Paliperidone effects on adolescent cocaine-induced offensive aggression [6] In a second experiment, adolescent low-dose cocaine-treated animals (N = 132) were assigned to one of four main groups based on the length of paliperidone exposure during development (i.e., 1, 2, 3, or 4 weeks). Within each group, animals were subdivided to vary the onset of drug treatment as outlined in Fig. 1. For example, animals receiving paliperidone for 1 week began treatment either at the start or 1 to 3 weeks after the start of cocaine treatment [i.e., 1 week (G1–G5), 2 weeks (G6–G8), 3 weeks (G9–G10), and 4 weeks (G11); Fig. 1]. Each subgroup contained equal number of animals (n = 12) resulting in a total of 11 treatment groups (G1–G11; Fig. 1). All groups received two daily i.p. injections: (1) low-dose cocaine (0.5 mg/kg) throughout adolescence (P27–P56) and (2) 0.1 mg/kg paliperidone or saline on days when paliperidone was not administered. The dose of paliperidone (0.1 mg/kg) was selected based on its anti-aggressive effects from Experiment 1. The day after the last injection (P57), experimental animals were tested for offensive aggression using the resident–intruder paradigm. As an aggressive control, a separate set of animals (n = 12) was treated with cocaine alone throughout puberty and tested for aggression in parallel with the above animals. Similarly, as a non-aggressive baseline behavioral control, a last set of hamsters was treated with saline alone throughout adolescence and tested for aggression. Schizophrenia-like hyperactivity model:Male Sprague-Dawley rats (200-250 g) were acclimated for 3 days. Paliperidone was dissolved in 0.5% carboxymethylcellulose sodium and administered via oral gavage (0.1 mg/kg, 0.3 mg/kg, 1 mg/kg) 30 minutes before subcutaneous injection of amphetamine (5 mg/kg). Record locomotor activity for 120 minutes [1]. - Novel object recognition (NOR) model:Male C57BL/6 mice (20-25 g) were orally administered Paliperidone (0.3 mg/kg, 1 mg/kg) daily for 7 days. On day 7, conduct NOR test: 10-minute familiarization with two identical objects, 1-hour interval, then 10-minute test with one familiar and one novel object. Calculate discrimination index [5]. - Neuroinflammation model:Male ICR mice (20-25 g) were intraperitoneally injected with LPS (5 mg/kg) to induce neuroinflammation. Paliperidone (0.5 mg/kg) was administered via intraperitoneal injection daily for 5 days. Euthanize mice to collect brain tissues for cytokine detection and immunohistochemistry [3] . |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute oral bioavailability of paliperidone following paliperidone administration is 28%. One week following administration of a single oral dose of 1 mg immediate-release 14C-paliperidone to 5 healthy volunteers, 59% (range 51% – 67%) of the dose was excreted unchanged into urine, 32% (26% – 41%) of the dose was recovered as metabolites, and 6% – 12% of the dose was not recovered. 487 L The absolute oral bioavailability of paliperidone following Invega administration is 28%. Administration of a 12 mg paliperidone extended-release tablet to healthy ambulatory subjects with a standard high-fat/high-caloric meal gave mean Cmax and AUC values of paliperidone that were increased by 60% and 54%, respectively, compared with administration under fasting conditions. Clinical trials establishing the safety and efficacy of Invega were carried out in subjects without regard to the timing of meals. While Invega can be taken without regard to food, the presence of food at the time of Invega administration may increase exposure to paliperidone. Based on a population analysis, the apparent volume of distribution of paliperidone is 487 L. The plasma protein binding of racemic paliperidone is 74%. Following a single dose, the plasma concentrations of paliperidone gradually rise to reach peak plasma concentration (Cmax) approximately 24 hours after dosing. The pharmacokinetics of paliperidone following Invega administration are dose-proportional within the available dose range. The terminal elimination half-life of paliperidone is approximately 23 hours. Steady-state concentrations of paliperidone are attained within 4-5 days of dosing with Invega in most subjects. The mean steady-state peak:trough ratio for an Invega dose of 9 mg was 1.7 with a range of 1.2-3.1. One week following administration of a single oral dose of 1 mg immediate-release (14)C-paliperidone to 5 healthy volunteers, 59% (range 51% - 67%) of the dose was excreted unchanged into urine, 32% (26% - 41%) of the dose was recovered as metabolites, and 6% - 12% of the dose was not recovered. Approximately 80% of the administered radioactivity was recovered in urine and 11% in the feces. Paliperidone is excreted in human breast milk. For more Absorption, Distribution and Excretion (Complete) data for Paliperidone (7 total), please visit the HSDB record page. Metabolism / Metabolites Although in vitro studies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, in vivo results indicate that these isozymes play a limited role in the overall elimination of paliperidone. Four primary metabolic pathways have been identified in vivo, none of which could be shown to account for more than 10% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission. Paliperidone does not undergo extensive metabolism and a significant portion of its metabolism occurs in the kidneys. Four primary metabolic pathways have been identified in vivo, none of which could be shown to account for more than 10% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission. Although in vitro studies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, in vivo results indicate that these isozymes play a limited role in the overall elimination of paliperidone. Paliperidone is a known human metabolite of risperidone. Biological Half-Life The terminal elimination half-life of paliperidone is approximately 23 hours. The median apparent half-life of paliperidone following Invega Sustenna single-dose administration over the dose range of 39 mg - 234 mg ranged from 25 days - 49 days. /Paliperidone palmitate/ The terminal elimination half-life of paliperidone is approximately 23 hours. Absorption:Oral bioavailability is 28% in humans; peak plasma concentration (Cmax) is reached at 24 hours post-oral administration (6 mg dose: Cmax=31 ng/mL) [2]. - Distribution:Volume of distribution (Vd) is 19.1 L/kg in humans; high blood-brain barrier penetration (brain/plasma concentration ratio=0.8-1.2) [2]. - Metabolism:Primarily metabolized in the liver via cytochrome P450 (CYP) 3A4 and uridine diphosphate glucuronosyltransferase (UGT) 1A4 to inactive metabolites; no significant first-pass metabolism [2,6]. - Excretion:59% of the dose is excreted in urine (40% as unchanged drug, 19% as metabolites), 32% in feces. Elimination half-life (t1/2) is 23-30 hours in humans [2]. - Plasma protein binding:Paliperidone has a plasma protein binding rate of 74% in human plasma [2] . |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Liver test abnormalities occur in up to 1% of patients receiving paliperidone, but similar rates have been reported with placebo therapy and with comparator agents. The ALT elevations are usually mild, transient and often resolve even without dose modification or drug discontinuation. There have been no published reports of clinically apparent liver injury with symptoms or jaundice attributed solely to paliperidone therapy, even with the long acting parenteral formulations. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although no data are available for the use of paliperidone during breastfeeding, it is the active metabolite of risperidone. Risperidone data indicate that the concentrations of paliperidone (9-hydroxyrisperidone) in breastmilk are low, and amounts ingested by the infant are small. A safety scoring system finds paliperidone possible to use cautiously during breastfeeding, although others do not recommend it. Because there is no published experience with paliperidone during breastfeeding and little long-term follow-up data, other agents may be preferred, especially while nursing a newborn or preterm infant. Because paliperidone is available only as long-acting products, timing of nursing with respect to doses would not be useful. Long-acting injectable formulations may continue to deliver small amounts to breastmilk for many months. Monitor breastfed infants for drowsiness, adequate growth and weight gain, jitteriness, tremors, and abnormal movements. ◉ Effects in Breastfed Infants No published information on paliperidone was found as of the revision date. However, limited data from the use of its parent drug, risperidone, during nursing indicate no short- or long-term adverse effects on the infant. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who were not treated with a second-generation antipsychotic (n = 818). Of the patients who were taking a second-generation antipsychotic drug, 60.4% were on more than one psychotropic. A review of the pediatric medical records, no adverse effects were noted among infants exposed or not exposed to second-generation antipsychotic monotherapy or to polytherapy. The number of women taking paliperidone was not reported. ◉ Effects on Lactation and Breastmilk Paliperidone has caused elevated prolactin serum levels, gynecomastia, and galactorrhea in patients taking the drug. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who had primarily diagnoses of major depressive disorder and anxiety disorders, most often treated with SSRI or SNRI antidepressants, but not with a second-generation antipsychotic (n = 818). Among women on a second-generation antipsychotic, 60.4% were on more than one psychotropic compared with 24.4% among women in the control group. Of the women on a second-generation antipsychotic, 59.3% reported “ever breastfeeding” compared to 88.2% of women in the control group. At 3 months postpartum, 23% of women on a second-generation antipsychotic were exclusively breastfeeding compared to 47% of women in the control group. The number of women taking paliperidone was not reported. Protein Binding The plasma protein binding of racemic paliperidone is 74%. Interactions Concurrent administration of carbamazepine and paliperidone decreased mean steady-state peak plasma concentrations and area under the concentration-time curves (AUCs) of paliperidone by approximately 37%. The manufacturer recommends reevaluating the dosage of paliperidone upon initiation of carbamazepine and increasing it, if necessary, based on clinical assessment. Upon discontinuance of carbamazepine, the dosage of paliperidone should also be reevaluated and decreased, if necessary. Potential pharmacologic interaction (possible disruption of body temperature regulation); use paliperidone with caution in patients concurrently receiving drugs with anticholinergic activity. Potential pharmacologic interaction /when used with othe CNS agents/ (additive CNS effects).Use with caution. Potential pharmacologic interaction (additive CNS effects). Avoid alcoholic beverages during paliperidone therapy. For more Interactions (Complete) data for Paliperidone (10 total), please visit the HSDB record page. Acute toxicity:LD50 is >1000 mg/kg (oral) in rats, >500 mg/kg (oral) in mice [6]. - Chronic toxicity:Rats administered Paliperidone (10 mg/kg/day, oral) for 6 months showed increased food intake and weight gain (15%), mild prolactin elevation, no significant liver/kidney toxicity or hematological abnormalities [6]. - Clinical side effects:Extrapyramidal symptoms (dystonia, parkinsonism) in 10-15% of patients; weight gain (20-25%), hyperprolactinemia (15-20%), sedation (8-12%), and QT interval prolongation (3-5%) [2,5]. - Drug-drug interaction:Inhibits CYP2D6, increasing plasma concentration of substrates (e.g., risperidone) by 30-35%; co-administration with CYP3A4 inhibitors (e.g., ketoconazole) increases paliperidone exposure by 60% [2,6] . |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antipsychotic Agent Invega (paliperidone) Extended-Release Tablets are indicated for the treatment of schizophrenia. The efficacy of Invega in schizophrenia was established in three 6-week trials in adults and one 6-week trial in adolescents, as well as one maintenance trial in adults. /Included in US product label/ Invega (paliperidone) Extended-Release Tablets are indicated for the treatment of schizoaffective disorder as monotherapy and an adjunct to mood stabilizers and/or antidepressant therap. The efficacy of Invega in schizoaffective disorder was established in two 6-week trials in adults. /Included in US product label/ Invega Sustenna (paliperidone palmitate) is indicated for the treatment of schizophrenia. Efficacy was established in four short-term studies and one longer-term study in adults. /Paliperidone palmitate; Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Invega (paliperidone) Extended-Release Tablets is not approved for the treatment of patients with dementia-related psychosis. /BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Invega Sustenna is not approved for use in patients with dementia-related psychosis. /Paliperidone palmitate/ Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been observed in patients receiving risperidone or paliperidone. Paliperidone is therefore contraindicated in patients with a known hypersensitivity to paliperidone, risperidone, or any ingredient in the paliperidone formulation. An increased incidence of adverse cerebrovascular events (cerebrovascular accidents and transient ischemic attacks), including fatalities, has been observed in geriatric patients with dementia-related psychosis treated with certain atypical antipsychotic agents (aripiprazole, olanzapine, risperidone) in placebo-controlled studies. The manufacturer states that paliperidone is not approved for the treatment of patients with dementia-related psychosis. For more Drug Warnings (Complete) data for Paliperidone (32 total), please visit the HSDB record page. Pharmacodynamics Paliperidone is an atypical antipsychotic developed by Janssen Pharmaceutica. Chemically, paliperidone is primary active metabolite of the older antipsychotic risperidone (paliperidone is 9-hydroxyrisperidone). The mechanism of action is unknown but it is likely to act via a similar pathway to risperidone. Because paliperidone is a major metabolite of risperidone (Ereshefsky and Lacombe 1993; Riedel et al. 2005), animals receiving risperidone are also exposed to paliperidone. However, most of risperidone is metabolized to paliperidone. Nevertheless, a small plasma concentration of risperidone was enough to attenuate the firing of 5-HT neurons. In summary, paliperidone and risperidone differentially affect the neuronal firing activity of 5-HT and NE neurons in vivo. The capacity of paliperidone to reverse the SSRI-induced inhibition of the NE neuronal firing rate, without the decreasing of the 5-HT neuronal activity like risperidone, suggests that paliperidone may be a very effective adjunct in SSRI-resistant depression.[5] In summary, the studies presented in this report examined the effects of acute and chronic paliperidone administration in a developmentally immature animal model of heightened offensive aggression. Our results indicate that short- and long- term exposure to paliperidone during a specific developmental period significantly reduces heightened levels of offensive aggression. Moreover, reductions in aggressive behavior occurred without concomitant alterations in non-aggressive behaviors supporting paliperidone’s targeted anti-aggressive effects. Our behavior results are novel and important in that they reveal the potential use of paliperidone in the treatment of a specific subtype of maladaptative aggression in emotionally disturbed youngsters in clinical and emergent settings.[6] Paliperidone is an atypical antipsychotic drug, active metabolite of risperidone, with dual dopamine/serotonin receptor antagonistic and neuroprotective activities [1,2,3,5]. - Mechanisms of action:Competitive antagonism of central D2/D3 and 5-HT2A/5-HT7 receptors (improving positive/negative symptoms of schizophrenia); inhibition of microglial activation and neuroinflammation; regulation of neuronal calcium homeostasis and promotion of BDNF-mediated neuroprotection [1,3,4,5]. - Indications:Schizophrenia (treatment of positive/negative symptoms and cognitive impairment); schizoaffective disorder [2,5]. - Administration:Oral extended-release tablets (3-12 mg once daily); intramuscular long-acting injection (234 mg every 4 weeks) for maintenance treatment [2]. - Clinical advantage:Long half-life supports once-daily dosing; lower risk of extrapyramidal symptoms than typical antipsychotics; improves cognitive function in schizophrenia patients [2,5]. - Caution:Avoid use in patients with QT interval prolongation, heart failure, or electrolyte disturbances; monitor prolactin levels and weight during long-term use [2,6] . |
| 分子式 |
C23H27FN4O3
|
|
|---|---|---|
| 分子量 |
426.48
|
|
| 精确质量 |
426.206
|
|
| 元素分析 |
C, 64.77; H, 6.38; F, 4.45; N, 13.14; O, 11.25
|
|
| CAS号 |
144598-75-4
|
|
| 相关CAS号 |
Paliperidone-d4; 1020719-55-4
|
|
| PubChem CID |
115237
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
612.3±65.0 °C at 760 mmHg
|
|
| 熔点 |
158-160°C
|
|
| 闪点 |
324.1±34.3 °C
|
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
|
| 折射率 |
1.692
|
|
| LogP |
1.52
|
|
| tPSA |
84.39
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
764
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC1C([H])=C([H])C2=C(C=1[H])ON=C2C1([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C2=C(C([H])([H])[H])N=C3[C@@]([H])(C([H])([H])C([H])([H])C([H])([H])N3C2=O)O[H])C([H])([H])C1([H])[H]
|
|
| InChi Key |
PMXMIIMHBWHSKN-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3
|
|
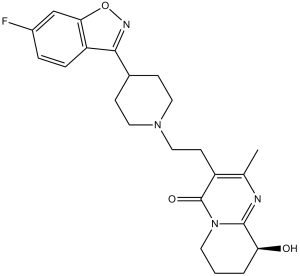
| 化学名 |
3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.5 mg/mL (1.17 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 5.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.5 mg/mL (1.17 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 5.0 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.5 mg/mL (1.17 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3448 mL | 11.7239 mL | 23.4478 mL | |
| 5 mM | 0.4690 mL | 2.3448 mL | 4.6896 mL | |
| 10 mM | 0.2345 mL | 1.1724 mL | 2.3448 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
An Observational Drug Utilization Study of Asenapine in the United Kingdom (P08308)
CTID: NCT01498770
Phase: Status: Completed
Date: 2022-02-04
 |
|---|
 |