| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HCV 1a (EC50 = 1 nM); HCV 1b (EC50 = 0.21 nM); SARS-CoV 3CLpro (IC50 = 1.31 μM)
Paritaprevir (also named ABT-450) targets hepatitis C virus (HCV) NS3/4A protease; EC50 values are 1.0 nM (genotype 1a), 0.21 nM (genotype 1b), 5.3 nM (genotype 2a), 19 nM (genotype 3a), 0.09 nM (genotype 4a), 0.69 nM (genotype 6a) against stable HCV replicons with NS3 protease [2] Paritaprevir is an NS3/4A protease inhibitor [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Paritaprevir(以前称为 ABT-450 和/或 Veruprevir)在结构上是一种酰基磺酰胺,是一种有效的非结构蛋白 3/4A (NS3/4A) 蛋白酶抑制剂,对 HCV 1a 的 EC50 值为 1 和 0.21 nM和1b,分别。 Abbott Laboratories 发现它在治疗丙型肝炎方面显示出有希望的结果。Paritaprevir 表现出针对 HCV GT1-4 和 GT6 的体外抗病毒活性(EC50 范围为 0.09 至 19 nM),针对 GT4a 的 EC50 为 0.09 nM。当与利托那韦和利巴韦林联合用药 12 周时,对于丙型肝炎病毒基因型 1 的患者,治疗后 24 周时的持续病毒学应答率估计为 95%。对帕立瑞韦治疗的耐药性并不常见,因为它的目标是结合位点,但已被认为是由于 NS3 中 155 和 168 位的突变而产生的。 Paritaprevir 是 Viekira Pak 和 Technivie 的成分。激酶测定:Paritaprevir 对 HCV GT1-4 和 GT6 具有体外抗病毒活性(EC50 范围,0.09 至 19 nM),对 GT4a 的 EC50 为 0.09 nM
1. Paritaprevir(ABT-450)在稳定HCV复制子实验中对不同基因型的HCV NS3/4A蛋白酶表现出强效抑制活性:针对1a、1b、2a、3a、4a、6a型的EC50值分别为1.0 nM、0.21 nM、5.3 nM、19 nM、0.09 nM、0.69 nM [2] 2. Paritaprevir(ABT-450)在1型HCV复制子的体外耐药筛选实验中,筛选出的最常见氨基酸变异位于NS3的155、156和168位;其中D168Y变异对Paritaprevir的耐药性最高(1a型耐药倍数219倍,1b型337倍) [2] |
| 体内研究 (In Vivo) |
帕立瑞韦、利托那韦、ombitasvir(一种 NS5A 蛋白抑制剂)和 dasabuvir(一种 NS5B 非核苷聚合酶抑制剂)联合或不联合 RBV 的组合已被批准用于治疗 HCV 基因型 1 感染[1]。
体内抗性发展的表型评估。[2] 对基线(首次给药前)和ABT-450/r单药治疗3天后的病毒分离株进行表型分析,以表征耐药变体的选择。通过计算3天单药治疗结束时EC50与基线相比的倍数变化来评估给药3天期间ABT-450表型耐药性的发展(表4)。24名患者中有13名(19名感染基因型1a的患者中有12名,5名感染基因基因型1b的患者中只有1名)的病毒载量水平足以(≥500IU/ml)在3天给药期结束时扩增靶基因(ABT-450/r 200/100-、100/100-和50/100mg治疗组分别有5、3和5名患者)。[2] 连续3天给予30mg/kg的帕替普韦可保护大鼠免受LPS诱导的ALI,这反映在肺系数(从0.75到0.64)和肺病理评分(从5.17到5.20)的变化上。此外,保护性粘附蛋白VE-cadherin和紧密连接蛋白claudin-5的水平升高,细胞质p-FOX-O1和核β-catenin和FOX-O1水平降低[3]。 1. 在一项针对1型HCV感染患者的3天单药治疗临床研究(II期)中,Paritaprevir(ABT-450)与利托那韦(CYP3A4抑制剂)联用;所有剂量组在3天给药结束时,HCV RNA平均最大下降幅度达4.02 log10 [2] 2. 在II/III期临床试验中,Paritaprevir/奥比他韦/利托那韦固定剂量组合联合达塞布韦(±利巴韦林),在慢性1型HCV感染成人患者(包括1a/1b型、代偿期肝硬化、肝移植受者、HIV-1合并感染)中实现了高比例的持续病毒学应答(SVR12) [1] 3. Paritaprevir(ABT-450)治疗患者中筛选出的耐药变异株为:1a型的R155K和D168V,1b型的D168V;与低剂量组相比,最高剂量组的耐药变异株筛选率显著降低 [2] |
| 酶活实验 |
Paritaprivir在体外对HCV GT1-4和GT6表现出抗病毒活性(EC50范围:0.09至19 nM),而其对GT4a的EC50为0.09 nM。
对一组抗性突变体的抗病毒活性。[2] 用于引入NS3基因中感兴趣突变的1a-H77和1b-Con1亚基因组复制子穿梭载体构建体与上述复制子细胞系构建体相似,但在这两种情况下,Neo基因都不存在,HCV NS2基因插入在EMCV IRES和NS3基因之间(图2B)。此外,1a-H77复制子构建体在NS3蛋白酶中编码E1202G的适应性突变被NS3解旋酶中编码P1496L的突变所取代。在NS3基因5′端上游62个核苷酸的NS2基因中引入AscI限制性位点,在NS3氨基酸251密码子后的NS3解旋酶结构域中引入BstBI限制性位点。这些限制性位点的引入不会导致基因型1a或1b复制子的氨基酸插入或改变。通过定点突变引入编码抗性相关变体的突变,并通过序列分析进行确认。亚基因组复制子RNA是通过将质粒DNA线性化,然后进行体外转录而产生的。将复制子RNA转染到Huh7衍生的细胞中,并使用如上所述的萤光素酶测定法测量Paritaprivir (ABT-450)对HCV复制子复制的抑制作用,除了细胞在裂解前孵育4天而不是3天。使用以下方程式计算复制效率作为野生型复制的百分比:100×[(突变型4天萤光素酶活性/野生型4天荧光素酶活性)/(突变型4-h萤光素酶活动/野生型4-h荧光素酶活性)][2]。 1. HCV NS3/4A蛋白酶抑制实验:采用携带1a、1b、2a、3a、4a、6a型NS3蛋白酶的稳定HCV复制子细胞系及瞬时复制子实验,评估Paritaprevir(ABT-450)的抑制活性;通过检测不同药物浓度下的病毒复制抑制率,确定各基因型对应的50%有效浓度(EC50) [2] 2. Paritaprevir耐药谱实验:对经Paritaprevir(ABT-450)处理的1型HCV复制子开展体外耐药筛选实验;鉴定NS3蛋白酶中的氨基酸变异,并通过检测EC50倍数变化量化关键变异株(如D168Y)的耐药水平 [2] |
| 细胞实验 |
细胞培养中的抗病毒活性。[2]
复制子细胞系在添加了100 IU/ml青霉素、100μg/ml链霉素和200μg/ml G418的Dulbecco改良Eagle培养基(DMEM)中维持,所有这些都来自Invitrogen,以及10%(体积/体积)的胎牛血清(FBS)。通过在含有5%FBS的相同培养基中在一系列ABT-450稀释液存在下孵育含复制子的细胞3天,然后使用萤光素酶测定系统测量萤火虫萤光素酶活性,来评估ABT-450的抑制作用。在测量人血浆存在下的抑制活性的试验中,培养基含有40%的人血浆和5%的FBS。计算每种化合物浓度对HCV RNA复制的抑制百分比,并使用非线性回归S型剂量反应变量斜率曲线拟合4参数逻辑斯谛方程和GraphPad Prism 4软件计算50%有效浓度(EC50)。通过3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑比色法测定ABT-450的细胞毒性。如上所述,使用非线性回归S型剂量反应可变斜率曲线拟合计算50%细胞毒性浓度(CC50)。 1. HCV复制子细胞实验:培养表达不同基因型(1a、1b、2a、3a、4a、6a)NS3蛋白酶的稳定HCV复制子细胞系,加入不同浓度的Paritaprevir(ABT-450);检测病毒复制水平以计算EC50值,同时采用瞬时复制子实验验证抑制活性 [2] 2. 耐药筛选细胞实验:将1型HCV复制子细胞暴露于Paritaprevir(ABT-450)以诱导耐药变异株;对NS3蛋白酶基因进行测序鉴定氨基酸突变,并评估含变异株的复制子的复制能力以确定耐药水平 [2] |
| 动物实验 |
In this study, 75 patients meeting all eligibility criteria and none of the exclusion criteria were randomized to receive various doses of ABT-450/r, dasabuvir, or ABT-072. Only data from the 24 patients treated with ABT-450/r are discussed in this report. Eligibility criteria for study M11-602 included the following: age of 18 to 65 years, body mass index (BMI) of ≥18 and <35 kg/m2, chronic HCV genotype 1 infection for at least 6 months prior to study enrollment, plasma HCV RNA level of ≥100,000 IU/ml at screening, liver biopsy within the past 3 years with histology consistent with HCV-induced liver damage, and no evidence of cirrhosis. Exclusion criteria included the following: liver biopsy with a METAVIR fibrosis score of 3 or 4, positive test result for hepatitis B surface antigen or anti-HIV antibodies, history of major depression within the 2 years prior to enrollment, history of disease precluding the use of pegIFN or RBV, and unresolved clinically significant diseases other than HCV.[2]
Patients were randomized to receive 1 of 3 doses of ABT-450/r (50/100 mg, 100/100 mg, or 200/100 mg) or placebo once daily (QD). Following 3 days of monotherapy, pegIFN alfa-2a at 180 μg/week and weight-based RBV at 1,000 to 1,200 mg/day were added, and the same dose of ABT-450/r or placebo was continued to complete a total of 12 weeks. At week 12, ABT-450/r or placebo was discontinued, and patients received pegIFN/RBV alone for up to 36 additional weeks.[2] 1. Clinical trial protocol (3-day monotherapy): HCV genotype 1-infected patients received Paritaprevir (ABT-450) coadministered with ritonavir at different doses for 3 days; plasma trough concentrations of Paritaprevir were measured, and HCV RNA levels were monitored to evaluate antiviral efficacy and resistance selection [2] 2. Phase II/III clinical trial protocol: Adults with chronic HCV genotype 1 infection were treated with Paritaprevir/ombitasvir/ritonavir (fixed-dose tablet) plus dasabuvir (± ribavirin); SVR12 (sustained virological response 12 weeks post-treatment) was the primary efficacy endpoint, and safety/tolerability was monitored [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Tmax of approximately 4 to 5 hours with a maximum concentration (Cmax) of 194 ng/mL. Following a single dose administration of 14C-paritaprevir co-dosed with 100 mg of ritonavir, approximately 88% of the radioactivity was recovered in feces with limited radioactivity (8.8%) in urine; unchanged paritaprevir accounted for 1.1% of the radioactivity in the feces and 0.05% in the urine. Volume of distribution at steady state is approximately 103 L. Metabolism / Metabolites Paritaprevir is predominantly metabolized by CYP3A4 and to a lesser extent by CYP3A5. Biological Half-Life 5.5 hr 1. Ritonavir (a cytochrome P450 3A4 inhibitor) markedly increased peak, trough, and overall plasma exposures of Paritaprevir (ABT-450) when coadministered [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Paritaprevir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is greater than 97% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. Some sources recommend against breastfeeding when paritaprevir is used with ribavirin. Ritonavir used as a booster has been studied in several studies of breastfeeding mothers. It is excreted into milk in measurable concentrations and low levels can be found in the blood of some breastfed infants. No reports of adverse reactions in breastfed infants have been reported. For more information, refer to the LactMed record on ritonavir. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 97 to 98.6% bound to human plasma proteins. 1. The combination regimen of Paritaprevir/ombitasvir/ritonavir plus dasabuvir was generally well tolerated in clinical trials; the most common adverse events included nausea, insomnia, asthenia, pruritus, other skin reactions, and fatigue [1] |
| 参考文献 |
[3]. Arch Pharm Res. 2023 Jun;46(6):564-572.
|
| 其他信息 |
Paritaprevir is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients. Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as paritaprevir. As a newer generation and directly acting HCV antiviral, paritaprevir products have better Sustained Virological Response (SVR) rates, higher barriers to resistance, fewer side effects, and a reduced pill burden compared to older agents such as [DB08873], [DB05521], [DB00008], [DB00022], and [DB00811]. By combining multiple antiretroviral medications into fixed dose products, the viral lifecycle can be targeted at multiple stages while simultaneously reducing the risk of developing resistant viral strains. Within Canada and the United States, paritaprevir is currently available in three fixed dose products: Viekira Pak (FDA), Technivie (FDA and Health Canada), and Holkira Pak (Health Canada). More specifically, paritaprevir prevents viral replication by inhibiting the NS3/4A serine protease of Hepatitis C Virus (HCV). Following viral replication of HCV genetic material and translation into a single polypeptide, Nonstructural Protein 3 (NS3) and its activating cofactor Nonstructural Protein 4A (NS4A) are responsible for cleaving genetic material into the following structural and nonstructural proteins required for assembly into mature virus: NS3, NS4A, NS4B, NS5A, and NS5B. By inhibiting viral protease NS3/4A, paritaprevir therefore prevents viral replication and function. In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Paritaprevir as a first line therapy option when used in combination with other antivirals for genotypes 1a, 1b, and 4. Depending on the genotype, Paritaprevir is often used in combination with other antivirals such as [DB09296], [DB09183], [DB00503], and [DB00811], with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality. Treatment with direct acting antivirals such as paritaprevir is associated with very minimal side effects, with the most common being headache and fatigue. Lack of significant side effects and short duration of therapy is a considerable advantage over older interferon-based regimens, which were limited by infusion site reactions, reduced blood count, and neuropsychiatric effects. Paritaprevir first came on the market as a fixed-dose combination product with [DB09296], [DB09183], and [DB00503] as the FDA-approved product Viekira Pak. First approved in December 2014, Viekira Pak is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis. Paritaprevir is also available as a fixed-dose combination product with [DB09296] and [DB00503] as the FDA- and Health Canada-approved product Technivie. First approved in July 2015, Technivie is indicated in combination with [DB00811] for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. In Canada, paritaprevir is also available as a fixed-dose combination product with [DB09296], [DB09183], and [DB00503] as the Health Canada-approved, commercially available product Holkira Pak. First approved in January 2015, Holkira Pak is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a with or without cirrhosis.
Paritaprevir is a Hepatitis C Virus NS3/4A Protease Inhibitor. The mechanism of action of paritaprevir is as a HCV NS3/4A Protease Inhibitor, and Organic Anion Transporting Polypeptide 1B1 Inhibitor, and Organic Anion Transporting Polypeptide 1B3 Inhibitor, and Breast Cancer Resistance Protein Inhibitor, and UGT1A1 Inhibitor, and P-Glycoprotein Inhibitor. Paritaprevir is an orally bioavailable, synthetic acylsulfonamide inhibitor of the hepatitis C virus (HCV) protease complex comprised of non-structural protein 3 and 4A (NS3/NS4A), with potential activity against HCV genotype 1. Upon administration, paritaprevir reversibly binds to the active center and binding site of the HCV NS3/NS4A protease and prevents NS3/NS4A protease-mediated polyprotein maturation. This disrupts both the processing of viral proteins and the formation of the viral replication complex, which inhibits viral replication in HCV genotype 1-infected host cells. NS3, a serine protease, is essential for the proteolytic cleavage of multiple sites within the HCV polyprotein and plays a key role during HCV ribonucleic acid (RNA) replication. NS4A is an activating factor for NS3. HCV is a small, enveloped, single-stranded RNA virus belonging to the Flaviviridae family, and infection is associated with the development of hepatocellular carcinoma (HCC). See also: Ombitasvir; Paritaprevir; Ritonavir (component of) ... View More ... Drug Indication When used within the fixed-dose combination product with [DB09296], [DB09183], and [DB00503] as the FDA-approved product Viekira Pak, paritaprevir is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis. When used within the fixed-dose combination product with [DB09296] and [DB00503] as the FDA- and Health Canada-approved product Technivie, paritaprevir is indicated in combination with [DB00811] for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. When used within the fixed-dose combination product with [DB09296], [DB09183], and [DB00503] as the Health Canada-approved, commercially available product Holkira Pak, paritaprevir is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a with or without cirrhosis. FDA Label Mechanism of Action Paritaprevir is a potent inhibitor of the NS3/4A serine protease of Hepatitis C Virus (HCV). Following viral replication of HCV genetic material and translation into a single polypeptide, Nonstructural Protein 3 (NS3) and its activating cofactor Nonstructural Protein 4A (NS4A) are responsible for cleaving it into the following structural and nonstructural proteins required for assembly into mature virus: NS3, NS4A, NS4B, NS5A, and NS5B. By inhibiting viral protease NS3/4A, paritaprevir therefore prevents viral replication and function. 1. Paritaprevir (ABT-450) is an NS3/4A protease inhibitor (direct-acting antiviral agent, DAA) for the treatment of HCV infection; rapid emergence of drug resistance is a major challenge for DAAs, leading to potential cross-resistance within the same drug class [2] 2. Paritaprevir is formulated as a fixed-dose tablet with ombitasvir (NS5A replication complex inhibitor) and ritonavir (CYP3A4 inhibitor), and is used in combination with dasabuvir (NS5B polymerase inhibitor) for chronic HCV genotype 1 infection; the regimen is interferon-free and approved in the USA (Viekira Pak™) and EU (Viekirax® + Exviera®) [1] 3. The 3-day monotherapy study of Paritaprevir (ABT-450) provided insights for subsequent clinical trials of combination therapy with other DAAs in HCV-infected patients [2] 4. Paritaprevir-containing combination therapy is effective in a broad range of HCV genotype 1-infected populations, including those with compensated cirrhosis, liver transplants, or HIV-1 co-infection [1] |
| 分子式 |
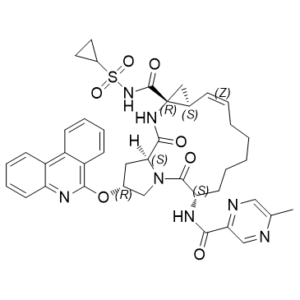
C40H43N7O7S
|
|---|---|
| 分子量 |
765.88
|
| 精确质量 |
765.294
|
| 元素分析 |
C, 62.73; H, 5.66; N, 12.80; O, 14.62; S, 4.19
|
| CAS号 |
1216941-48-8
|
| 相关CAS号 |
Paritaprevir dihydrate;1456607-71-8
|
| PubChem CID |
45110509
|
| 外观&性状 |
White to off-white powder
|
| LogP |
6.346
|
| tPSA |
208.5
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
55
|
| 分子复杂度/Complexity |
1600
|
| 定义原子立体中心数目 |
5
|
| SMILES |
S(C1([H])C([H])([H])C1([H])[H])(N([H])C([C@@]12C([H])([H])[C@@]1([H])C([H])=C([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[C@@]([H])(C(N1C([H])([H])[C@@]([H])(C([H])([H])[C@]1([H])C(N2[H])=O)OC1C2=C([H])C([H])=C([H])C([H])=C2C2=C([H])C([H])=C([H])C([H])=C2N=1)=O)N([H])C(C1C([H])=NC(C([H])([H])[H])=C([H])N=1)=O)=O)(=O)=O
|
| InChi Key |
UAUIUKWPKRJZJV-QPLHLKROSA-N
|
| InChi Code |
InChI=1S/C40H43N7O7S/c1-24-21-42-33(22-41-24)35(48)43-32-16-6-4-2-3-5-11-25-20-40(25,39(51)46-55(52,53)27-17-18-27)45-36(49)34-19-26(23-47(34)38(32)50)54-37-30-14-8-7-12-28(30)29-13-9-10-15-31(29)44-37/h5,7-15,21-22,25-27,32,34H,2-4,6,16-20,23H2,1H3,(H,43,48)(H,45,49)(H,46,51)/b11-5-/t25-,26-,32+,34+,40-/m1/s1
|
| 化学名 |
(1S,4R,6S,7Z,14S,18R)-N-cyclopropylsulfonyl-14-[(5-methylpyrazine-2-carbonyl)amino]-2,15-dioxo-18-phenanthridin-6-yloxy-3,16-diazatricyclo[14.3.0.04,6]nonadec-7-ene-4-carboxamide
|
| 别名 |
ATB450; ABT 450; ABT-450; Veruprevir; Paritaprevir; Brand name: VIEKIRA PAK; 1216941-48-8; Veruprevir; ABT-450; Veruprevir anhydrous; ABT450; 1221573-85-8; Paritaprevir(ABT-450);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (2.72 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.72 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3057 mL | 6.5284 mL | 13.0569 mL | |
| 5 mM | 0.2611 mL | 1.3057 mL | 2.6114 mL | |
| 10 mM | 0.1306 mL | 0.6528 mL | 1.3057 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Variability at signature NS5A amino acid positions across HCV GT4 subtypes.Antimicrob Agents Chemother.2015 Nov;59(11):6807-15. |
|---|
Phylogenetic analysis of HCV GT4 baseline sequences.Antimicrob Agents Chemother.2015 Nov;59(11):6807-15. |