| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
WEE1 (IC50 = 24 nM); Myt1 (IC50 = 72 nM); Chk1 (IC50 = 3.433 μM)
|
|---|---|
| 体外研究 (In Vitro) |
在七分之七的癌细胞系中,PD0166285 (0.5 μM) 显着抑制辐射诱导的 Cdc2 Tyr-15 和 Thr-14 磷酸化[1]。在 p53 突变体 HT29 细胞和 E6 转染、p53 缺失的卵巢癌细胞系 PA-1 中,PD0166285 使辐射诱导的细胞杀伤变得敏感;然而,在 p53 野生型 PA-1 细胞中,这种效应不太明显。 PD0166285 可逆转放射诱导的 G2 期停滞,有丝分裂细胞群显着增加[1]。 PD0166285的敏感性增强比为1.23,作为放射增敏剂,使细胞对辐射引起的细胞死亡更加敏感[1]。
许多癌症细胞缺乏功能性p53提供了治疗靶点。预计缺乏p53的细胞不会表现出G(1)检查点,并且将依赖于G(2)检查点在经历有丝分裂之前允许DNA修复。我们假设G(2)检查点消除剂可以通过去除保护这些细胞免受DNA损伤过早有丝分裂的唯一检查点,优先杀死p53不活跃的癌症细胞。由于Wee1激酶通过其对Cdc2的抑制性磷酸化在维持G(2)阻滞中起着至关重要的作用,我们开发了一种高通量大规模筛选方法,并将其用于筛选Wee1抑制剂的化学文库。已鉴定出一类吡啶并嘧啶分子,PD0166285,在纳摩尔浓度下抑制Wee1。在细胞水平上,0.5μMPD0166285在所测试的七个癌症细胞系中的七个细胞系中,显著抑制照射诱导的Tyr-15和Thr-14的Cdc2磷酸化。如生化标志物和荧光激活细胞分选仪分析所示,PD0166285消除了辐射诱导的G(2)阻滞,并显著增加了有丝分裂细胞群。在生物学上,PD0166285充当放射增敏剂,使细胞对辐射诱导的细胞死亡敏感,如标准克隆形成试验所示,敏感性增强率为1.23。这种放射增敏活性是p53依赖性的,在p53失活细胞中具有更高的疗效。因此,G(2)检查点消除剂代表了一类新型抗癌药物,通过诱导过早有丝分裂来增强传统癌症治疗的细胞杀伤。[1] PD0166285在体外由P-糖蛋白转运,但不由BCRP转运[2] 研究PD0166285易位的CETA在体外显示了P-gp的转运活性,但没有BCRP的转运活性。在所有表达BCRP的细胞系中,均未观察到易位(图3)。与BCRP相反,发现表达Abcb1a和ABCB1的细胞系都能运输PD0166285,而亲本猪细胞系则不能。同样,在P-gp抑制剂zosuquidar存在的情况下,易位的丧失进一步证实了P-gp是观察到的PD0166285转运的原因。 |
| 体内研究 (In Vivo) |
P-gp,而非BCRP,限制了体内PD0166285的脑渗透[2]
进行了与上述AZD1775类似的药代动力学实验,研究PD0166285的脑渗透。在这个实验中,Abcb1a/b−/−和Abcb1a/b的脑水平都增加了大约5倍;Abcg2-/-小鼠与野生型小鼠的比较(图4b)。因此,这种效应似乎完全是由P-gp引起的,因为当Abcg2-/-也不存在时,没有观察到PD0166285脑水平进一步升高。脑水平的这些差异也反映在脑血浆比率上,因为血浆水平在所有遗传背景下都是相似的。总之,这些结果表明,PD0166285在体内的脑渗透受P-gp的限制,但不受BCRP的限制。 |
| 酶活实验 |
浓度平衡迁移分析[2]
如前所述,使用500 nM的Wee1抑制剂进行浓度平衡转运分析(CETAs),并使用5μM的zosuquidar或elacridar阻断转运。为了制备CETA样品以供后续HPLC分析,将培养基样品与两倍体积的乙腈混合。离心后,将上清液用水稀释3倍,使用GraceSmart RP18 5μm柱(150×2mm)(Grace,Deerfield,IL)通过与紫外检测器连接的高效液相色谱法(HPLC)测量AZD1775或PD0166285的浓度。使用等度条件在340 nm处检测到AZD1775,其中45%乙腈在0.1%(v/v)甲酸水溶液中以0.2 mL/min的流速输送。PD0166285在360 nm处使用相同的柱检测到,该柱用甲醇和0.1%(v/v)甲酸水溶液梯度洗脱,流速为0.2 mL/min,范围为30%至70%。 |
| 细胞实验 |
蛋白质印迹分析[1]
细胞类型:人和小鼠癌细胞系(HCT116、HT29、DLD-1、HCT8、H460、HeLa、C 26)。 测试浓度:0.5 μM。 孵化持续时间:4小时。 实验结果:抑制Cdc2Y15和CdcT14磷酸化。 |
| 动物实验 |
Animal/Disease Models: Wild-type, Abcg2-/-, Abcb1a/b-/- and Abcb1a/b;Abcg2-/- FVB mice[2].
Doses: 5 mg/kg. Route of Administration: IV. Experimental Results: Cmax is about 400 ng/mL. P-gp, but not BCRP, limited the brain penetration of PD0166285. Pharmacokinetic studies [2] We used wildtype, Abcg2−/−, Abcb1a/b−/− and Abcg2;Abcb1a/b−/− FVB mice. PD0166285 (5 mg/kg) and AZD1775 (20 mg/kg) were administered i.v. in DMSO. Blood was collected by cardiac puncture 1 h after injection under isoflurane anesthesia, followed by brain tissue collection. Plasma was obtained by centrifugation (5 min, 5000 rpm, 4 °C). Brains were weighed and homogenized using a FastPrep®-24 in 1% (w/v) bovine serum albumin in water. All samples were stored at −20 °C until analysis. AZD1775 and PD0166285 were extracted using diethyl ether and AZD8055 was used as internal standard. Organic phases were separated and dried by vacuum. Samples were reconstituted in methanol:water (20:80 v/v) and measured in an LC-MS/MS setup consisting of an Ultimate 3000 LC System and an API 4000 mass spectrometer. Separation was performed on a ZORBAX Extend-C18 column. Mobile phase A (0.1% formic acid in water) and B (methanol) was used in a 5 min gradient from 30 to 95%B maintained for 3 min followed by re-equilibration at 30%B. Multiple reaction monitoring (MRM) ion traces were 501.5 / 442.4 (AZD1775) and 512.2 / 438.9 (PD0166285) and 466.2 / 450.1 (AZD8055). |
| 药代性质 (ADME/PK) |
Pharmacokinetic experiments using wildtype and ABC transport knockout mice sampled for brain and plasma at 1 h after drug administration clearly show that these same transporters are responsible for the very low brain penetration of the Wee1 inhibitors in vivo (Fig. 4). Notably, in the absence of these transporters the brain-plasma ratio of both agents was remarkably high (approximately 25 for AZD1775 and 6 for PD0166285), whereas in wild type mice AZD1775 and PD0166285 could only achieve a brain-plasma ratio of 1.0 and 1.2, respectively.
|
| 参考文献 | |
| 其他信息 |
PD-0166285 is a small molecule drug with a maximum clinical trial phase of I.
Introduction Wee1 is an important kinase involved in the G2 cell cycle checkpoint and frequently upregulated in intracranial neoplasms such as glioblastoma (GBM) and diffuse intrinsic pontine glioma (DIPG). Two small molecules are available that target Wee1, AZD1775 and PD0166285, and clinical trials with AZD1775 have already been started. Since GBM and DIPG are highly invasive brain tumors, they are at least to some extent protected by the blood-brain barrier (BBB) and its ATP-binding cassette (ABC) efflux transporters. Methods We have here conducted a comprehensive set of in vitro and in vivo experiments to determine to what extent two dominant efflux transporters in the BBB, P-gp (ABCB1) and BCRP (ABCG2), exhibit affinity towards AZD1775 and PD0166285 and restrict their brain penetration. Results Using these studies, we demonstrate that AZD1775 is efficiently transported by both P-gp and BCRP, whereas PD0166285 is only a substrate of P-gp. Nonetheless, the brain penetration of both compounds was severely restricted in vivo, as indicated by a 5-fold (PD0166285) and 25-fold (AZD1775) lower brain-plasma ratio in wild type mice compared to Abcb1a/b;Abcg2-/- mice. Conclusion The brain penetration of these Wee1 inhibitors is severely limited by ABC transporters, which may compromise their clinical efficacy against intracranial neoplasms such as DIPG and GBM. [2] In summary, targeting Wee1 to treat intracranial neoplasms holds promise since Wee1 is overexpressed in various glioma types and several clinical trials have been started. However, since gliomas are highly invasive and thus to a considerable extent protected by the BBB, using a Wee1 inhibitor with sufficient brain penetration capacity is pivotal to the success of this treatment strategy. We demonstrate that both available Wee1 inhibitors, AZD1775 and PD0166285, are efficient substrates of ABC transporters in the BBB and it is therefore not very likely that they will be able to exhibit efficacy in patients.[2] |
| 分子式 |
C26H27CL2N5O2
|
|
|---|---|---|
| 分子量 |
512.4309
|
|
| 精确质量 |
511.154
|
|
| 元素分析 |
C, 60.94; H, 5.31; Cl, 13.84; N, 13.67; O, 6.24
|
|
| CAS号 |
185039-89-8
|
|
| 相关CAS号 |
PD0166285 dihydrochloride;212391-63-4; PD0166285;185039-89-8; 1933496-20-8 (HCl hydrate)
|
|
| PubChem CID |
5311382
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
665.3±65.0 °C at 760 mmHg
|
|
| 闪点 |
356.2±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.637
|
|
| LogP |
5.09
|
|
| tPSA |
70.6
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
35
|
|
| 分子复杂度/Complexity |
719
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
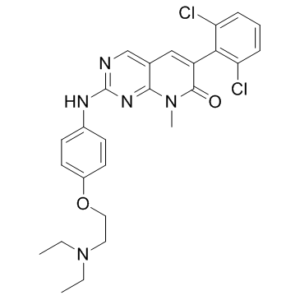
ClC1C=CC=C(C=1C1=CC2=CN=C(NC3C=CC(=CC=3)OCCN(CC)CC)N=C2N(C)C1=O)Cl
|
|
| InChi Key |
IFPPYSWJNWHOLQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31)
|
|
| 化学名 |
6-(2,6-dichlorophenyl)-2-((4-(2-(diethylamino)ethoxy)phenyl)amino)-8-methylpyrido[2,3-d]pyrimidin-7(8H)-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.17 mg/mL (4.23 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9515 mL | 9.7574 mL | 19.5149 mL | |
| 5 mM | 0.3903 mL | 1.9515 mL | 3.9030 mL | |
| 10 mM | 0.1951 mL | 0.9757 mL | 1.9515 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。