| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
MEK1 (Ki = 1 nM); MEK2 (Ki = 1 nM); MEK1 (IC50 = 0.33 nM)
|
|---|---|
| 体外研究 (In Vitro) |
PD0325901 显示出比另一种 MEK 抑制剂 CI-1040 更高的渗透性。与 CI-1040 相比,PD0325901 应该能够实现更高的全身暴露。 [1] PD0325901 针对活化的 MEK1 和 MEK2 的 Kiapp 为 1 nM,并且针对纯化的 MEK 具有令人难以置信的特异性和效力。 [2] 就其对 ERK1 和 ERK2 磷酸化的细胞作用而言,PD0325901 的效力比 CI-1040 大约强 500 倍,并且表现出亚纳摩尔活性。 [2] PD0325901 阻止黑色素瘤细胞系增殖。 K2 细胞和 TPC-1 细胞均受到 PD0325901 的生长抑制,GI50 值分别为 11 nM 和 6.3 nM。 [3]在极低浓度 (10 nM) 下,PD0325901 可显着抑制含有 BRAF 突变的 PTC 细胞的生长,而仅轻微促进含有 RET/PTC1 重排的 PTC 细胞的生长。在许多 PTC 细胞系中,PD0325901 有效抑制 ERK1/2 磷酸化。 [3]
|
| 体内研究 (In Vivo) |
显然,PD0325901 比 CI-1040 具有更强的效力。给药后 24 小时,单次口服剂量的 PD0325901 (25 mg/kg) 显着降低 ERK 的磷酸化。然而,pERK 水平仅在高得多的剂量(150 mg/kg)时被 CI-1040 抑制,并且在治疗后 24 小时恢复正常。 [2]因此,25 mg/kg/天,而不是 PD0325901 和 CI-1040 的 900 mg/kg/天,是产生 70% 完全肿瘤反应发生率(C26 模型)所需的剂量。多种人类肿瘤异种移植物已显示 PD 0325901 的抗癌活性。 [2]注射携带 BRAF 突变的 PTC 细胞的小鼠在口服 PD0325901 一周(20–25 mg/kg/天)后并未出现肿瘤。 [3]与对照组相比,具有 RET/PTC1 重排的 PTC 中原位肿瘤的平均肿瘤体积减少了 58%。总之,具有 BRAF 突变的 PTC 细胞比具有 RET/PTC1 重排的 PTC 细胞对 PD0325901 更敏感。 [3]
|
| 酶活实验 |
含有 p44MAP 激酶的谷胱甘肽 S-转移酶融合蛋白 (GST-MAPK) 和含有 p45MEK 的谷胱甘肽 S-转移酶蛋白 (GST-MEK) 的存在用于测量 32P 掺入髓磷脂碱性蛋白 (MBP) 的情况。测定溶液的终浓度为 100 mL,由 20 mM HEPES、pH 7.4、10 mM MgCl2、1 mM MnCl2、1 mM EGTA、50 mM [gamma-32P]ATP、10 mg GST-MEK、0.5 mg 组成GST-MAPK 和 40 毫克 MBP。 20分钟后加入三氯乙酸终止反应,然后将混合物通过GF/C滤垫过滤。 1205 Betaplate 用于测量过滤垫上保留的 32P 量。为了确定剂量反应曲线,在一定剂量范围内评估 PD0325901。
|
| 细胞实验 |
将 PTC 细胞 ((1 × 104) 置于含有 1 mL 培养基的 24 孔板中,并在 37 °C 下孵育 4 天。第 0 天,用不同浓度的 MEK 抑制剂 PD0325901 处理细胞一式三份。第 2 天测试 GI50 或每天检测细胞生长曲线,每孔加入溶解在 0.8% NaCl 溶液中的 5 mg/mL MTT(0.2 mL)。将细胞与 MTT 在 37 °C 下孵育 3 小时。随后从孔中吸出并倾倒。使用 Synergy HT 多重检测酶标仪,将染色细胞溶解在 0.5 mL DMSO 中,并测量其在 570 nm 处的吸光度。对于 GI50,细胞生长计算为 100 × (T − T0 )/(C − T0),其中 T 是实验开始后 48 小时后用抑制剂处理的孔的光密度,T0 是零时间时的光密度,C 是仅使用 DMSO 的光密度控制井。
|
| 动物实验 |
Mice (10–14 per group) are s.c. cocktail-anesthetized. Inoculated into the thyroid gland are K2 and TPC-1 cells that have been stably infected with a retrovirus expressing luciferase (5×105 cells in 5 μL of RPMI1640 medium), and the mice are then checked every week by Xenogen using Living Image 3.0 software to look for tumor development. PD0325901 is dissolved in 80 mM citric buffer (pH 7) using a sonicator one week after inoculation, and mice are then given daily oral gavage doses of 20 to 25 mg/kg for three weeks (5 days in a row). Only mice with tumor burden or a 20% body weight loss are sacrificed. The formula (V=length×width×depth) is used to calculate tumor volume (V), which is measured using calipers. Mice under control are only given 80 mM citric buffer (pH 7). All in vivo experiments are done at least twice.
|
| 毒性/毒理 (Toxicokinetics/TK) |
In terms of safety, the most commons any-grade AEs observed were dermatitis acneiform (42.3%), fatigue (32.4%), and diarrhea (26.8%). The most common grade ≥3 AE was thrombocytopenia and platelet count decrease (5.6%).
Overall, 87.3% of the treatment-related AEs were from lifirafenib and 88.7% were from mirdametinib. Serious AEs related to lifirafenib treatment were seen in 42.3% of patients and SAEs from mirdametinib were observed in 14.1%. DLT TEAEs occurred in 9.9% of patients overall. These TEAEs lead to dose modification in 57.7%, discontinuation of treatment in 5.6%, and death in 5.6%. Based on these findings, Solomon concluded that the low-grade serous ovarian cancer, NSCLC, and endometrial cancer patient population who harbor BRAF and KRAS mutations may be sensitive to the combination of lifirafenib and mirdametinib. Further study on the benefit-risk profile of the combination is warranted. https://www.targetedonc.com/view/lifirafenib-plus-mirdametinib-shows-tolerable-safety-in-braf-kras-mutant-advanced-solid-tumors |
| 参考文献 | |
| 其他信息 |
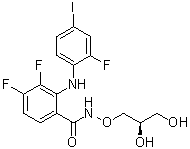
PD 0325901 is a hydroxamic acid ester that is benzhydroxamic acid (N-hydroxybenzamide) in which the hydroxamic acid group has been converted to the corresponding 2,3-dihydroxypropyl ester and in which the benzene ring has been substituted at position 2 by a (2-fluoro-4-iodophenyl)amino group and at positions 3 and 4 by fluorines (the R enantiomer). It has a role as an EC 2.7.12.2 (mitogen-activated protein kinase kinase) inhibitor and an antineoplastic agent. It is a hydroxamic acid ester, a secondary amino compound, a member of monofluorobenzenes, an organoiodine compound, a member of propane-1,2-diols and a difluorobenzene.
PD-0325901 has been used in trials studying the treatment and basic science of Melanoma, Solid Tumour, Solid Tumors, Advanced Cancer, and Breast Neoplasms, among others. Mirdametinib is an orally bioavailable, synthetic organic molecule targeting mitogen-activated protein kinase kinase (MAPK/ERK kinase or MEK) with potential antineoplastic activity. Upon administration, mirdametinib selectively binds to and inhibits MEK, which may result in the inhibition of the phosphorylation and activation of MAPK/ERK and the inhibition of tumor cell proliferation. The dual specific threonine/tyrosine kinase MEK is a key component of the RAS/RAF/MEK/ERK signaling pathway that is frequently activated in human tumors. A novel series of benzhydroxamate esters derived from their precursor anthranilic acids have been prepared and have been identified as potent MEK inhibitors. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide, CI-1040, was the first MEK inhibitor to demonstrate in vivo activity in preclinical animal models and subsequently became the first MEK inhibitor to enter clinical trial. CI-1040 suffered however from poor exposure due to its poor solubility and rapid clearance, and as a result, development of the compound was terminated. Optimization of the diphenylamine core and modification of the hydroxamate side chain for cell potency, solubility, and exposure with oral delivery resulted in the discovery of the clinical candidate N-(2,3-dihydroxy-propoxy)-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-benzamide PD 0325901.[1] Papillary thyroid carcinomas (PTC) are the most common type of thyroid malignancy. Most PTC carry one of the two mutations, RET/PTC rearrangement or BRAF mutation. Both mutations are able to activate the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling transduction pathway leading to cellular proliferation, differentiation, and apoptosis. PD0325901 is a specific MEK1/2 inhibitor and therefore is a promising drug to treat thyroid cancers with either RET/PTC or BRAF mutation. In this study we tested the effects of PD0325901 on PTC cells harboring either mutation in vitro by growth curves and Western blots and in vivo using a murine orthotopic xenograft model. We found that 50% growth inhibition (GI(50)) by PD0325901 was 11 nmol/L for the PTC cells with the RET/PTC1 rearrangement and 6.3 nmol/L for PTC cells with a BRAF mutation, with both concentrations readily achievable in serum. After 1 week of oral administration of PD0325901 (20-25 mg/kg/day) in mice, no tumor growth was detected in mice inoculated with PTC cells bearing a BRAF mutation. For PTC with the RET/PTC1 rearrangement, the average tumor volume of the orthotopic tumor was reduced by 58% as compared with controls. In conclusion, our data suggested that PTC cells carrying a BRAF mutation were more sensitive to PD0325901 than were PTC cells carrying the RET/PTC1 rearrangement. Our findings support the clinical evaluation of PD0325901 for patients with PTC and potentially other carcinomas with BRAF mutations.[3] |
| 分子式 |
C16H14F3IN2O4
|
|---|---|
| 分子量 |
482.19
|
| 精确质量 |
481.995
|
| 元素分析 |
C, 39.85; H, 2.93; F, 11.82; I, 26.32; N, 5.81; O, 13.27
|
| CAS号 |
391210-10-9
|
| 相关CAS号 |
Mirdametinib;391210-10-9
|
| PubChem CID |
9826528
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.8±0.1 g/cm3
|
| 熔点 |
112-114ºC
|
| 闪点 |
1.645
|
| 折射率 |
1.645
|
| LogP |
6.16
|
| tPSA |
90.82
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
465
|
| 定义原子立体中心数目 |
1
|
| SMILES |
O=C(C1=CC=C(C(F)=C1NC2=CC=C(I)C=C2F)F)NOC[C@H](O)CO
|
| InChi Key |
SUDAHWBOROXANE-SECBINFHSA-N
|
| InChi Code |
InChI=1S/C16H14F3IN2O4/c17-11-3-2-10(16(25)22-26-7-9(24)6-23)15(14(11)19)21-13-4-1-8(20)5-12(13)18/h1-5,9,21,23-24H,6-7H2,(H,22,25)/t9-/m1/s1
|
| 化学名 |
N-[(2R)-2,3-dihydroxypropoxy]-3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzamide
|
| 别名 |
Mirdametinib; PD 0325901; PD-0325901; PD-325901; N-[(2R)-2,3-dihydroxypropoxy]-3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]benzamide;PD0325901; PD-325901; PD 325901; PD325901; 391210-10-9; Mirdametinib; (R)-N-(2,3-Dihydroxypropoxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)benzamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.31 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.31 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.31 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% PEG 400+5% Tween 80+ddH2O: 10mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0739 mL | 10.3694 mL | 20.7387 mL | |
| 5 mM | 0.4148 mL | 2.0739 mL | 4.1477 mL | |
| 10 mM | 0.2074 mL | 1.0369 mL | 2.0739 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05054374 | Active Recruiting |
Drug: Mirdametinib Drug: Fulvestrant |
Breast Cancer Solid Carcinoma |
Memorial Sloan Kettering Cancer Center |
September 14, 2021 | Phase 1 Phase 2 |
| NCT03962543 | Active Recruiting |
Drug: Mirdametinib (PD-0325901) oral capsule or dispersible tablet |
Neurofibromatosis Type 1 (NF1) Plexiform Neurofibroma |
SpringWorks Therapeutics, Inc. | September 29, 2019 | Phase 2 |
| NCT05580770 | Recruiting | Drug: Mirdametinib Drug: BGB-3245 |
Advanced Solid Tumor | SpringWorks Therapeutics, Inc. | February 3, 2023 | Phase 1 Phase 2 |
| NCT04923126 | Recruiting | Drug: Mirdametinib | Low-Grade Glioma Recurrent Low-Grade Glioma |
St. Jude Children's Research Hospital |
February 3, 2023 | Phase 1 Phase 2 |
| NCT03905148 | Recruiting | Drug: Lifirafenib Drug: mirdametinib |
Solid Tumor, Adult | BeiGene | May 1, 2019 | Phase 1 |
PD0325901 inhibits PTC cell growthin vitro.Mol Cancer Ther.2010 Jul;9(7):1968-76.
|
PD0325901 suppresses the expression of p-ERK1/2 and induces apoptosis in PTC cells.Mol Cancer Ther.2010 Jul;9(7):1968-76. |
PD0325901 inhibits tumor growth in mice.Mol Cancer Ther.2010 Jul;9(7):1968-76. |