| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
FGFR1 (IC50 = 52.4 nM)
|
|---|---|
| 体外研究 (In Vitro) |
PD166866抑制FGFR1扩增的乳腺癌症细胞中的细胞增殖。
PD166866诱导FGFR1扩增的乳腺癌症细胞自噬。
靶向自噬提高了PD166866的抗癌效果。
PD166866通过抑制Akt/mTOR通路诱导自噬。[3]
体外活性:PD166866 抑制人全长 FGFR-1 酪氨酸激酶,IC50 值为 52.4 nM,是 FGFR-1 的 ATP 竞争性抑制剂。 PD 166866 是表达内源性 FGFR-1 的 NIH 3T3 细胞和过表达人 FGFR-1 酪氨酸激酶的 L6 细胞中 FGFR 自磷酸化的有效抑制剂。 PD 166866 还抑制 L6 细胞中 bFGF 诱导的 44-和 42-kDa (ERK 1/2) 丝裂原激活蛋白激酶亚型的酪氨酸磷酸化。每日将 PD 166866 以 1 至 100 nM 的浓度暴露于 L6 细胞,会连续 8 天对 bFGF 刺激的细胞生长产生浓度相关的抑制,IC50 值为 24 nM。激酶测定:PD166866 是一种新结构的酪氨酸激酶抑制剂 6-芳基-吡啶并[2,3-d]嘧啶的成员,是一种新型纳摩尔强效选择性 FGFR(成纤维细胞生长因子-1)小分子抑制剂受体)酪氨酸激酶抑制剂,IC50 为 52.4 nM。 PD 166866 是表达内源性 FGFR-1 的 NIH 3T3 细胞和过表达人 FGFR-1 酪氨酸激酶的 L6 细胞中 FGFR 自磷酸化的有效抑制剂。 PD 166866 还抑制 L6 细胞中 bFGF 诱导的 44-和 42-kDa (ERK 1/2) 丝裂原激活蛋白激酶亚型的酪氨酸磷酸化。 PD 166866 具有作为抗增殖/抗血管生成剂的潜在用途,可用于治疗肿瘤生长和动脉粥样硬化斑块的新血管形成等治疗目标。细胞测定:将 PD 166866 溶解在 DMSO 中。每天将 PD 166866 或载体(0.5% DMSO,终浓度)与 25 ng/mL bFGF 一起添加到一式三份的细胞培养物中,以刺激 FGF 驱动的生长。在一些实验中,每天将 PD 166866 与 30 ng/mL PDGF-BB 一起添加到一式三份的细胞培养物中,以刺激 PDGF 驱动的生长。药物暴露后第 1、3、6 或 8 天通过 Coulter 计数测量细胞数。 通过直接合成,我们发现了一种小分子,它是人成纤维细胞生长因子-1受体(FGFR)酪氨酸激酶的纳摩尔抑制剂。PD166866是酪氨酸激酶抑制剂新结构类别6-芳基吡啶并[2,3-d]嘧啶的成员,通过筛选具有测量蛋白酪氨酸激酶活性的测定的化合物库进行鉴定。PD 166866抑制人全长FGFR-1酪氨酸激酶,IC50值为52.4+/-0.1nM,并进一步被表征为FGFR-1的ATP竞争性抑制剂。相比之下,PD 166866在高达50微M的浓度下对c-Src、血小板衍生生长因子受体β、表皮生长因子受体或胰岛素受体酪氨酸激酶或丝裂原活化蛋白激酶、蛋白激酶c和CDK4没有影响。PD166866是表达内源性FGFR-1的NIH 3T3细胞和过表达人FGFR-1酪氨酸激酶的L6细胞中碱性成纤维细胞生长因子(bFGF)介导的受体自磷酸化的强效抑制剂,证实了酪氨酸激酶介导的机制。PD 166866还抑制了L6细胞中bFGF诱导的44和42 kDa(ERK 1/2)丝裂原活化蛋白激酶亚型的酪氨酸磷酸化,可能是通过抑制bFGF刺激的FGFR-1酪氨酸激酶活化。PD 166866分别不抑制血管平滑肌、A431或NIHIR细胞中的血小板衍生生长因子、表皮生长因子或胰岛素刺激的受体自磷酸化,进一步支持其对FGFR-1的特异性。此外,PD 166866以1至100 nM的浓度每天暴露于L6细胞,导致bFGF刺激的细胞生长连续8天受到浓度相关的抑制,IC50值为24 nM。相比之下,PD 166866对血小板源性生长因子BB刺激的L6细胞生长或血清刺激的血管平滑肌细胞增殖几乎没有影响。最后,发现PD 166866是人类胎盘培养动脉片段微血管生长(血管生成)的强效抑制剂。这些结果强调了PD 166866的发现,PD 166866是一种新的纳摩尔强效和选择性的FGFR-1酪氨酸激酶小分子抑制剂,可能用作肿瘤生长和动脉粥样硬化斑块新生血管等治疗靶点的抗增殖/抗血管生成剂。[2] 癌症约占每年诊断的癌症妇科病例的30%,是女性癌症相关死亡率的主要原因。FGFR1的扩增在乳腺癌中经常被观察到,并且与不良预后有关。尽管FGFR长期以来被认为是抗癌药物靶点,并且一组FGFR拮抗剂目前正在进行临床试验,但FGFR拮抗药治疗下的确切细胞反应仍不清楚。在这里,我们发现PD166866,一种FGFR1选择性抑制剂,在FGFR1扩增的乳腺癌症细胞系中抑制增殖并触发失活。值得注意的是,我们证明PD166866在FGFR1扩增的乳腺癌症细胞系中诱导自噬,而Atg5敲低对自噬的阻断进一步增强了PD166 866的抗增殖活性。此外,机制研究表明,PD166866通过抑制Akt/mTOR信号通路诱导自噬。总之,本研究为FGFR拮抗剂抗肿瘤活性的分子机制提供了新的见解,并可能进一步帮助基于FGFR的药物发现。 |
| 体内研究 (In Vivo) |
为了扩展我们的体外研究结果,通过皮下注射稳定表达shAtg5的MDA-MB-134亚系建立了小鼠肿瘤异种移植物模型,并用PD166866治疗小鼠。尽管前五天没有观察到差异,但与由模拟载体转染细胞组成的肿瘤相比,由Atg5沉默细胞形成的肿瘤显示出明显降低的生长速率(图3E-F)。此外,Ki-67免疫染色显示,当Atg5表达受到抑制时,肿瘤异种移植物中增殖细胞的数量大大减少,如图3G所示。这些结果表明,阻断自噬改善了PD166866在FGFR1扩增的乳腺癌症中的抗增殖作用[3]。
|
| 酶活实验 |
聚ADP核糖聚合酶(PARP)的免疫定位[1]
PARP酶在DNA断裂时被激活。PARP的免疫定位如先前发表的那样进行。简而言之,HeLa细胞用PD166866处理24小时,去除生长培养基,用PBS洗涤细胞,在25°C下固定1小时,加入新鲜制备的副甲醛溶液(4%的PBS溶液)。再次用PBS洗涤样品,在黑暗中阻断内源性氧化酶2分钟。随后用PBS进一步洗涤,在25°C下阻断非特异性位点1小时。PARP的证据是利用针对N端蛋白水解片段的多克隆抗体进行免疫定位。在4°C下孵育16小时后,通过二次抗兔抗体显示免疫反应。用PBS彻底洗涤后,将样品在ABC溶液中孵育30分钟。最终,加入DAB(3,3'-二氨基联苯胺),在黑暗中孵育样品10分钟。再次洗涤样品,密封平板,准备进行显微镜观察。 PD166866是一种新型 FGFR(成纤维细胞生长因子-1 受体)酪氨酸激酶小分子抑制剂,IC50 为 52.4 nM。它属于一种新结构的酪氨酸激酶抑制剂,6-芳基-吡啶并[2,3-d]嘧啶。在过表达人 FGFR-1 酪氨酸激酶的 L6 细胞和表达内源性 FGFR-1 的 NIH 3T3 细胞中,PD 166866 是 FGFR 自磷酸化的强抑制剂。此外,PD 166866 还可防止 L6 细胞 bFGF 诱导的 44-和 42-kDa (ERK 1/2) 丝裂原激活蛋白激酶亚型的酪氨酸磷酸化。对于肿瘤生长和动脉粥样硬化斑块新生血管等治疗靶点,PD 166866 具有作为抗增殖/抗血管生成剂的潜在应用。 |
| 细胞实验 |
HeLa 细胞暴露于PD166866一整天后,除去生长培养基,使用 PBS 洗涤细胞,并使用最近制备的多聚甲醛溶液(PBS 中的 4%)将它们在 25°C 下固定一小时。再次用 PBS 清洗样品后,在避光条件下将内源氧化酶封闭两分钟。之后,进行更多的 PBS 洗涤,并将未指定的位点在 25°C 下封闭一小时。通过使用多克隆抗体靶向 N 端蛋白水解片段进行免疫定位,证明了 PARP。 4°C 孵育 16 小时后,通过抗兔二抗进行免疫反应。将样品在 PBS 中彻底清洗,然后在溶液 ABC 中孵育半小时。添加 DAB(3,3'-二氨基联苯胺)后,将样品在黑暗中孵育 10 分钟。再次洗涤后,将板密封并准备用于显微镜检查。
HeLa细胞的生长和维持[1] 细胞在DMEM(Dulbecco's Modified Eagle's Medium-高糖)中维持,补充新生牛血清[终浓度(f.c.)10%]、青霉素-链霉素(10000 U/ml)和谷氨酰胺(2 mM);培养基的pH值为7.2,在37°C和5%CO2气氛中孵育。细胞在融合时常规传代。 细胞活力评估和脂质过氧化试验[1] 通过比色Mosmann测定法评估细胞存活率,该测定法是一种测量线粒体损伤水平的定量方法。MTT[3-(4,5-二甲基噻唑-2-基)-2,5-二芬太尼溴化四唑]是一种黄色的水溶性盐,可转化为由活细胞线粒体中存在的活性脱氢酶形成的不溶性紫色盐。在570nm处测量的吸光度值提供了活细胞的数量。细胞存活数据通过标准实验室方案用台盼蓝进行活体染色进行验证 使用商业试剂盒评估膜水平的氧化应激。简而言之,该测定基于对细胞内丙二醛(MDA)形成的定量分析,MDA来源于多不饱和脂肪酸的分解。MDA分子与显色化合物(N-甲基-2-苯基吲哚)反应,从而形成稳定的发色团。586nm处的吸光度直接转化为细胞内MDA浓度。 DNA片段的TUNEL检测和分析[1] 内源性DNA酶的激活是细胞死亡导致单链缺口形成并最终导致DNA断裂的后果之一。DNA断裂可以通过原位标记来证明。细胞核被渗透,加入荧光dUTP,末端脱氧核苷酸转移酶与糖磷酸骨架中断的核苷酸结合。荧光强度提供了DNA损伤的定性概念。 |
| 动物实验 |
Female nude mice
20 mg/kg i.p. Tumor xenograft model [3] Healthy female nude mice (6–8 weeks, 18–20 g) were injected subcutaneously with mock vector- or shAtg5-overexpressed MDA-MB-134 cells (5 × 106 cells/mouse). When the tumors reached 100 mm3 in volume, mice were peritoneally treated with 20 mg/kg PD166866. The tumor volume was measured every 5 days and animals were sacrificed after 25 days. Tumors were dissected and frozen in liquid nitrogen or fixed in formalin immediately. |
| 毒性/毒理 (Toxicokinetics/TK) |
Cytotoxicity of PD166866 on HeLa cells in culture [1]
We explored the dose/response effect of HeLa cells exposed to a relatively broad range of PD166866 concentrations (0.1 - 50 μM). Cells were treated for 24 hours with the drug and their vitality assessed by the MTT assay. A significant reduction of vital cells can be monitored already at 2.5 μM concentration (Figure 1, left panel). The loss of viability seems to stabilize at 25 μM (about 25% survival) with no further decrease at a 50 μM concentration of drug. This result may indicate the presence of a cell subpopulation, intrinsically resistant to the drug. This result was confirmed by vital cell count with trypan blue (only the data obtained at 2.5 μM of drug is shown; Figure 1, right panel). The negative effect of PD166866 on the cell growth was already observed in a previous works performed on 3T6 cells: a stabilized murine fibroblast line. The results presented here validate those already published and, as far as cell survival is concerning, no difference can be monitored on HeLa in comparison to 3T6 cells in matching experiments also run in this work (not shown). Interestingly, as observed in a former study, HeLa cells showed a significantly higher sensitivity than murine cells towards resveratrol, a natural product showing both cytotoxic and antiviral properties. One way to rationalize this data is that the cellular/molecular target of the two drugs could be different. |
| 参考文献 | |
| 其他信息 |
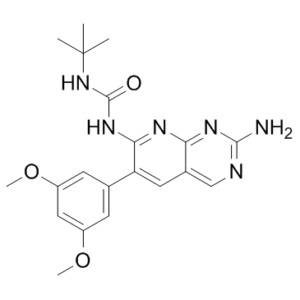
PD-166866 is a member of the class of pyridopyrimidines that is pyrido[2,3-d]pyrimidine substituted by an amino group at position 2, 3,5-dimethoxyphenyl group at position 6, and by a (tert-butylcarbamoyl)nitrilo group at position 7. It is a selective ATP competitive inhibitor of the human fibroblast growth factor-1 receptor (FGFR1) tyrosine kinase with an IC50 of 52.4 nM. It has a role as an apoptosis inducer, an antineoplastic agent, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor and an angiogenesis inhibitor. It is a dimethoxybenzene, a pyridopyrimidine, a member of ureas, a biaryl and a primary arylamine.
Background: Many experimental data evidence that over-expression of various growth factors cause disorders in cell proliferation. The role of the Fibroblast Growth Factors (FGF) in growth control is indisputable: in particular, FGF1 and its tyrosine kinase receptor (FGFR1) act through a very complex network of mechanisms and pathways. In this work we have evaluated the antiproliferative activity effect of PD166866, a synthetic molecule inhibiting the tyrosin kinase action of FGFR1. Methods: Cells were routinely grown in Dulbecco Modified Eagle's medium supplemented with newborn serum and a penicillin-streptomycin mixture.Cell viability was evaluated by Mosmann assay and by trypan blue staining. DNA damage was assessed by in situ fluorescent staining with Terminal Deoxynucleotidyl Transferase dUTP nick end labeling (TUNEL assay).Assessment of oxidative stress at membrane level was measured by quantitative analysis of the intra-cellular formation of malonyl-dialdheyde (MDA) deriving from the decomposition of poly-unsaturated fatty acids.The expression of Poly-ADP-Ribose-Polymerase (PARP), consequent to DNA fragmentation, was evidenced by immuno-histochemistry utilizing an antibody directed against an N-terminal fragment of the enzyme. Results: The bioactivity of the drug was investigated on Hela cells. Cytoxicity was assessed by the Mosmann assay and by vital staining with trypan blue. The target of the molecule is most likely the cell membrane as shown by the significant increase of the intracellular concentration of malonyl-dihaldheyde. The increase of this compound, as a consequence of the treatment with PD166866, is suggestive of membrane lipoperoxidation. The TUNEL assay gave a qualitative, though clear, indication of DNA damage. Furthermore we demonstrate intracellular accumulation of poly-ADP-ribose polymerase I. This enzyme is a sensor of nicks on the DNA strands and this supports the idea that treatment with the drug induces cell death. Conclusions: Data presented in this work show that PD166866 has clear antiproliferative effects. The negative control of cell proliferation may be exerted through the activation of the apoptotic pathway. The results of experiments addressing this specific point, such as: evaluation of DNA damage, lipoperoxidation of the cell membrane and increase of expression of PARP, an enzyme directly involved in DNA repair. Results suggest that cells exposed to PD16866 undergo apoptosis. However, concomitant modes of cell death cannot be ruled out. The possible use of this drug for therapeutic purposes is discussed.[1] Through direct synthetic efforts, we discovered a small molecule that is a nanomolar inhibitor of the human fibroblast growth factor-1 receptor (FGFR) tyrosine kinase. PD 166866, a member of a new structural class of tyrosine kinase inhibitors, the 6-aryl-pyrido[2,3-d]pyrimidines, was identified by screening a compound library with assays that measure protein tyrosine kinase activity. PD 166866 inhibited human full-length FGFR-1 tyrosine kinase with an IC50 value of 52.4 +/- 0.1 nM and was further characterized as an ATP competitive inhibitor of the FGFR-1. In contrast, PD 166866 had no effect on c-Src, platelet-derived growth factor receptor-beta, epidermal growth factor receptor or insulin receptor tyrosine kinases or on mitogen-activated protein kinase, protein kinase C and CDK4 at concentrations as high as 50 microM. PD 166866 was a potent inhibitor of basic fibroblast growth factor (bFGF)-mediated receptor autophosphorylation in NIH 3T3 cells expressing endogenous FGFR-1 and in L6 cells overexpressing the human FGFR-1 tyrosine kinase, confirming a tyrosine kinase-mediated mechanism. PD 166866 also inhibited bFGF-induced tyrosine phosphorylation of the 44- and 42-kDa (ERK 1/2) mitogen-activated protein kinase isoforms in L6 cells, presumably via inhibition of bFGF-stimulated FGFR-1 tyrosine kinase activation. PD 166866 did not inhibit platelet-derived growth factor, epidermal growth factor or insulin-stimulated receptor autophosphorylation in vascular smooth muscle, A431 or NIHIR cells, respectively, further supporting its specificity for the FGFR-1. In addition, daily exposure of PD 166866 to L6 cells at concentrations from 1 to 100 nM resulted in a concentration-related inhibition of bFGF-stimulated cell growth for 8 consecutive days with an IC50 value of 24 nM. In contrast, PD 166866 had little effect on platelet-derived growth factor-BB-stimulated growth of L6 cells or serum-stimulated vascular smooth muscle cell proliferation. Finally, PD 166866 was found to be a potent inhibitor of microvessel outgrowth (angiogenesis) from cultured artery fragments of human placenta. These results highlight the discovery of PD 166866, a new nanomolar potent and selective small molecule inhibitor of the FGFR-1 tyrosine kinase with potential use as antiproliferative/antiangiogenic agent for such therapeutic targets as tumor growth and neovascularization of atherosclerotic plaques. [2] Breast cancer, representing approximately 30% of all gynecological cancer cases diagnosed yearly, is a leading cause of cancer-related mortality for women. Amplification of FGFR1 is frequently observed in breast cancers and is associated with poor prognosis. Though FGFRs have long been considered as anti-cancer drug targets, and a cluster of FGFR antagonists are currently under clinical trials, the precise cellular responses under the treatment of FGFR antagonists remains unclear. Here, we show that PD166866, an FGFR1-selective inhibitor, inhibits proliferation and triggers anoikis in FGFR1-amplified breast cancer cell lines. Notably, we demonstrate that PD166866 induces autophagy in FGFR1-amplified breast cancer cell lines, while blockage of autophagy by Atg5 knockdown further enhances the anti-proliferative activities of PD166866. Moreover, mechanistic study reveals that PD166866 induces autophagy through repressing Akt/mTOR signaling pathway. Together, the present study provides new insights into the molecular mechanisms underlying the anti-tumor activities of FGFR antagonists, and may further assist the FGFRs-based drug discovery.[3] |
| 分子式 |
C20H24N6O3
|
|
|---|---|---|
| 分子量 |
396.44
|
|
| 精确质量 |
396.191
|
|
| 元素分析 |
C, 60.59; H, 6.10; N, 21.20; O, 12.11
|
|
| CAS号 |
192705-79-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5328127
|
|
| 外观&性状 |
white solid powder
|
|
| 密度 |
1.277g/cm3
|
|
| 折射率 |
1.643
|
|
| LogP |
3.545
|
|
| tPSA |
128.5
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
545
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
C1=C2C(N=C(NC(=O)NC(C)(C)C)C(C3C=C(OC)C=C(OC)C=3)=C2)=NC(N)=N1
|
|
| InChi Key |
NHJSWORVNIOXIT-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H24N6O3/c1-20(2,3)26-19(27)25-17-15(8-12-10-22-18(21)24-16(12)23-17)11-6-13(28-4)9-14(7-11)29-5/h6-10H,1-5H3,(H4,21,22,23,24,25,26,27)
|
|
| 化学名 |
1-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butylurea
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (2.52 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (2.52 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 5% DMSO+40% PEG 300+5% Tween 80+50% ddH2O: 0.5mg/mL 配方 4 中的溶解度: 3.33 mg/mL (8.40 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 需要超声助溶并加热至 40°C。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5224 mL | 12.6122 mL | 25.2245 mL | |
| 5 mM | 0.5045 mL | 2.5224 mL | 5.0449 mL | |
| 10 mM | 0.2522 mL | 1.2612 mL | 2.5224 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Assessment of cell survival after treatment with PD166866.
Intracellular concentration of malonyl-dihaldehyde (MDA) after treatment with PD166866.J Exp Clin Cancer Res.2009 Dec 11;28:151. |
An extensive DNA damage is caused by treatment with PD166866.J Exp Clin Cancer Res.2009 Dec 11;28:151. |
Accumulation Poly-ADP-Ribose-Polymerase (PARP) in cells treated with PD166866 evidenced by imuno-histochemistry.J Exp Clin Cancer Res.2009 Dec 11;28:151. |