| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
MEK1 (IC50 = 2-7 μM); MEK2 (IC50 = 50 μM); ERK1; ERK2; Autophagy
|
|---|---|
| 体外研究 (In Vitro) |
PD98059 抑制基础 MEK1 或通过将残基 218 和 222 处的丝氨酸更改为谷氨酸 (MEK-2E) 而产生的部分激活的 MEK,IC50 为 2 M。MAPK 同源物 JNK 和 P38 不会以任何方式被 PD98059 抑制。 Raf 激酶、cAMP 依赖性激酶、蛋白激酶 C、v-Src、表皮生长因子 (EGF) 受体激酶、胰岛素受体激酶、PDGF 受体激酶和磷脂酰肌醇 3-激酶只是 PD98059 不具备的一些其他激酶抑制。 PD98059 的 IC50 分别为 ~10 μM 和 ~7 μM,可阻断 PDGF 刺激的 MAPK 激活和胸苷掺入 3T3 细胞。 [1] PD98059 的 IC50 为 4 μM,这使得它有效抑制 Raf 或 MEK 激酶激活 MEK1,IC50 为 50 μM,这使得它弱抑制 Raf 激活 MEK2。在 KB 和 PC12 细胞以及 Swiss 3T3 细胞中,PD98059 不会阻止 MEK 同源物 MKK4 和 RK 激酶的激活,这些激酶参与应激和白细胞介素 1 刺激的激酶级联反应。 [2] PD98059完全阻止神经生长因子(NGF)引起的PC12细胞的分化,而不影响细胞活力。 [3] PD98059 在 RANKL 存在的情况下对 RAW264.7 细胞增殖的剂量依赖性抑制导致培养物中 TRAP 阳性细胞的明显减少。 [4]
|
| 体内研究 (In Vivo) |
对小鼠进行治疗 在局灶性脑缺血前 30 分钟给予 PD98059 以防止损伤时,梗塞体积会减少。 [5]根据胰腺湿重和组织学,在每小时一次的雨蛙素注射前 30 分钟给予 PD98059 预处理(每次静脉注射 10 毫克/千克),持续三小时可显着降低雨蛙素诱发的急性胰腺炎的严重程度。 [6]在角叉菜胶后一小时给予小鼠 PD98059(10 毫克/千克),可减少所有测量到的炎症参数。 [7]
本研究的目的是评估丝裂原活化蛋白激酶1-3MAPK3/MAPK1)在小鼠急性肺部炎症模型中的作用。将卡拉胶注射到小鼠胸膜腔内引发急性炎症反应,其特征是:胸膜腔内积聚含有大量中性粒细胞(PMNs)的液体,PMNs浸润肺组织,随后粘附分子表达(I-CAM和P-选择素),脂质过氧化,肿瘤坏死因子α(TNF-α)和白细胞介素-1β(IL-1β)的产生增加。此外,角叉菜胶诱导肺细胞凋亡(Bax和Bcl-2表达)以及硝基酪氨酸形成、NF-kB激活和pJNK表达,通过肺组织的免疫组织化学分析以及肺部炎症和组织损伤的程度(组织学评分)确定。角叉菜胶1小时后施用PD98059,一种MAPK3/MAPK1抑制剂(10mg/kg),导致所有测量的炎症参数降低。因此,基于这些发现,我们提出MAPK3/MAPK1信号通路的抑制剂,如PD98059,可能可用于治疗各种炎症性疾病[7]。 |
| 酶活实验 |
MEK和ERK活性的体外抑制[12]
对将PD98059用作信号转导研究工具的兴趣源于它抑制MEK但不抑制其他激酶的特异性(Alessi等人,1995;Dudley等人,1995)。激酶级联分析用于量化PD98059对MEK的抑制作用(图2)。该检测使用突变的、组成型激活的MEK形式来磷酸化和激活ERK1,进而磷酸化MBP。因此,MEK的活性可以通过测量ERK1或MBP来监测。。。 PD98059[2-(2'-氨基-3'-甲氧基苯基)-恶萘-4-酮]是一种黄酮类化合物,是丝裂原活化蛋白激酶(MEK)的强效抑制剂。浓度/=10微M时具有弱细胞生长抑制作用。在体内将培养物暴露于95%)(IC50=1μM)。在添加2,3,7,8-四氯二苯并对二恶英(TCDD)时或添加前48小时用>/=1μM的PD98059处理培养物,以浓度依赖的方式抑制了诱导的稳态CYP1A1、CYP1B1和NQO1 mRNA的积累。通过蔗糖梯度离心和电泳迁移率变化测定,在添加TCDD之前向大鼠肝细胞质中添加PD98059抑制了TCDD结合(IC50=4μM)和芳基烃受体(AHR)转化(IC50=1μM)。黄酮和黄烷酮是PD98059的两个密切相关的结构类似物,它们通过TCDD抑制AHR转化,IC50值与PD98059。然而,这两种类似物在抑制MEK方面都不如PD98059有效(两者的IC50约为190μM)。这些结果表明,PD98059是AHR的配体,在通常用于抑制MEK和导致MEK激活的信号传导过程的浓度下,它作为AHR拮抗剂发挥作用。[12] 为了测量 32P 掺入髓磷脂碱性蛋白 (MBP) 中,使用含有 44-kDa MAPK (GST-MAPK) 或 45-kDa MEK (GST-MEK1) 的谷胱甘肽 S-转移酶 (GST) 融合蛋白。测定在 50 μL 溶液中进行,该溶液含有 10 μg GST-MEK1、0.5 μg GST-MAPK 和 40 μg MBP,以及 50 mM Tris,pH 7.4、10 mM MgCl2、2 mM EGTA 和 10 μM [γ-32P]ATP。 30°C 孵育 15 分钟后,添加 Laemmli SDS 样品缓冲液可终止反应。通过使用 SDS/10% PAGE,磷酸化的 MBP 得以分离。 |
| 细胞实验 |
将细胞以 10,000–20,000/mL 的密度接种到多孔板中进行单层生长。 48小时后,向细胞生长培养基中添加不同浓度的PD98059,然后再孵育3天。胰蛋白酶孵育后,从孔中提取细胞并使用库尔特计数器进行计数。将细胞以每皿 5,000-10,000 个细胞的密度接种到 35 毫米培养皿中,生长培养基中含有所需浓度的 PD98059 和 0.3% 琼脂,以便在软琼脂中生长。生长 7-10 天后,使用解剖显微镜对可见菌落进行手动计数。
细胞系和细胞培养。[11] 在含有10%热灭活FCS的RPMI 1640中培养三种人AML细胞系。OCI-AML-3和MOLM-13细胞具有野生型p53,而HL-60中的p53因TP53的大量缺失而失活。在对数生长期收获细胞系,以2×105个细胞/mL的密度接种,并暴露于Nutlin-3a和/或PD98059。在涉及Nutlin-3a和PD98059组合的实验中,在20μmol/L PD98059存在或不存在的情况下,用0、1、2.5、5和10μmol/L的Nutlin-3a和0、0.4、1、2和4μmol/L的MOLM-13细胞处理OCI-AML-3和HL-60细胞。将这两种试剂同时添加到细胞中,并培养24小时。通过三次台盼蓝染料计数来评估细胞存活率,不包括细胞。实验至少做了两次。 实时定量PCR。[11] 用20μmol/LPD98059和/或5μmol/L Nutlin-3a处理OCI-AML-3细胞3小时。使用RNeasy Mini Kit从细胞中制备RNA,并使用1μg总RNA的随机六聚体(SuperScript III第一链合成SuperMix)生成第一链cDNA。使用TaqMan基因表达测定p21、Mdm2、Noxa、ABL和18S的mRNA表达水平。使用ABI Prism 7500序列检测系统和TaqMan Universal Master Mix进行定量实时PCR。反应首先在95°C下保持10分钟,然后在95°℃下保持15秒,在60°C下持续1分钟。使用两个18S探针(Hs99999901_s1)和p21探针(Hs00355782_m1)。PCR产物的特异性通过ABI SDS 2.0软件的熔解曲线分析得到证实。基于测试样品和对照未处理细胞之间的CT值差异计算相对表达水平。使用方程Etarget(CTtesttarget−CTcontrol target)/Eref(CTtestref−CTcontrolref)将其归一化为18S的表达水平。实时PCR实验进行了三次。 |
| 动物实验 |
Male Sprague–Dawley rats with acute pancreatitis
10 mg/kg Injection i.v. PD98059 (MEK1 Inhibitor) has been shown to act in vivo as a highly selective inhibitor of MEK1 activation and the MAP kinase cascade. In the present study, we have investigated the effects of PD98059, on the development of non-septic shock caused by zymosan in mice. Mice received either intraperitoneally zymosan (500mg/kg, administered i.p. as a suspension in saline) or vehicle (0.25ml/mouse saline). PD98059 (10mg/kg) was administered 1 and 6h after zymosan administration i.p. Organ failure and systemic inflammation in mice was assessed 18h after administration of zymosan and/or PD98059. Treatment of mice with PD98059 attenuated the peritoneal exudation and the migration of polymorphonuclear cells caused by zymosan. PD98059 also attenuated the lung, liver and pancreatic injury and renal dysfunction caused by zymosan as well as the increase of TNF-alpha and IL-1beta plasma levels caused by zymosan. Immunohistochemical analysis for inducible nitric oxide synthase (iNOS), nitrotyrosine, poly(ADP-ribose) (PAR), ICAM-1, P-selectin, Bax, Bcl-2 and FAS-ligand revealed positive staining in pancreatic and intestinal tissue obtained from zymosan-injected mice. The degree of staining for nitrotyrosine, iNOS, PAR, ICAM-1, P-selectin, Bax, Bcl-2 and FAS-ligand were markedly reduced in tissue sections obtained from zymosan-injected mice, which had received PD98059. Moreover treatment of mice with PD98059 (10mg/kg) attenuated the NF-kappaB activation and mitogen-activated protein kinases (MAPK) expression induced by zymosan injection. In addition, administration of zymosan caused a severe illness in the mice characterized by a systemic toxicity, significant loss of body weight and a 60% of mortality at the end of observation period. Treatment with PD98059 significantly reduced the development of systemic toxicity, the loss in body weight and the mortality (20%) caused by zymosan. This study provides evidence that PD98059 attenuates the degree of zymosan-induced non-septic shock in mice. Pharmacol Res. 2010 Feb;61(2):175-87. PD98059 (2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one) (Sigma-Aldrich, USA) was dissolved in 75% DMSO. The PD98059 (2.5 mcg/5 mcl, i.t.) was single or repeated preemptively administered 16 h and 1 h before CCI and then once daily for 7 days (the administration was according to our previous paper [17], [25]). The Vehicle-treated CCI-exposed rats received 75% DMSO according to the same schedule. There was no significant difference in pain behavior between no-treated and V(DMSO)-treated CCI-exposed rats. This method of PD98059 or vehicle administration was used throughout the study and is referred to in the text as “repeated administration”. At day 7th after CCI 30 min after PD98059 administration tactile allodynia was measured using von Frey test and thermal hyperalgesia was conducted using cold plate test. Additionally, at day 7th after CCI the vehicle-treated and PD98059-treated rats received a single i.t. vehicle, morphine (2.5 mcg/5 mcl) or buprenorphine (2.5 mcg/5 mcl) injection 30 min after PD98059, and then 30 min later the von Frey and/or cold plate tests were repeated. Since the dose of morphine 2.5 mcg/5 mcl in naïve rats produced maximal analgesic effect in tail-flick test. We have used lower dose of morphine for co-administration experiments, so that we would be able to observe the possible enhancement of opioid effectiveness. The vehicle-treated and PD98059-treated naïve rats (uninjured rats) received a single i.t. vehicle, morphine (0.5 mcg/5 mcl) or buprenorphine (2.5 mcg/5 mcl) injection 30 min after PD98059, and then 30 min later the tail flick test was performed (S1 Table and S1 Fig). https://pmc.ncbi.nlm.nih.gov/articles/PMC4591269/#sec002 |
| 参考文献 |
[1]. Proc Natl Acad Sci U S A . 1995 Aug 15;92(17):7686-9. [2]. J Biol Chem . 1995 Nov 17;270(46):27489-94. [3]. J Biol Chem . 1995 Jun 9;270(23):13585-8. [4]. J Biol Chem . 2002 Dec 6;277(49):47366-72. [5]. Proc Natl Acad Sci U S A . 1999 Oct 26;96(22):12866-9. [6]. Pancreas . 2002 Oct;25(3):251-9. [7]. Int J Immunopathol Pharmacol . 2009 Oct-Dec;22(4):937-50. [8]. Melanoma Res . 2001 Feb;11(1):11-9. [9]. Int Immunopharmacol . 2007 Jan;7(1):36-45. [10]. Int J Cancer . 2003 Nov 10;107(3):478-85. |
| 其他信息 |
2-(2-amino-3-methoxyphenyl)chromen-4-one is a member of the class of monomethoxyflavones that is 3'-methoxyflavone bearing an additional amino substituent at position 2'. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor and a geroprotector. It is a monomethoxyflavone and an aromatic amine.
PD-98059 is an inhibitor of MAP-kinase kinase activation. 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one has been reported in Pestalotiopsis neglecta with data available. MEK Inhibitor PD-98059 is a cell-permeable, selective mitogen-activated protein (MAP) kinase inhibitor which exhibits activity through the inhibition of the phosphorylation and activation of MAP kinase. Treatment of cells with a variety of growth factors triggers a phosphorylation cascade that leads to activation of mitogen-activated protein kinases (MAPKs, also called extracellular signal-regulated kinases, or ERKs). We have identified a synthetic inhibitor of the MAPK pathway. PD 098059 [2-(2'-amino-3'-methoxyphenyl)-oxanaphthalen-4-one] selectively inhibited the MAPK-activating enzyme, MAPK/ERK kinase (MEK), without significant inhibitory activity of MAPK itself. Inhibition of MEK by PD 098059 prevented activation of MAPK and subsequent phosphorylation of MAPK substrates both in vitro and in intact cells. Moreover, PD 098059 inhibited stimulation of cell growth and reversed the phenotype of ras-transformed BALB 3T3 mouse fibroblasts and rat kidney cells. These results indicate that the MAPK pathway is essential for growth and maintenance of the ras-transformed phenotype. Further, PD 098059 is an invaluable tool that will help elucidate the role of the MAPK cascade in a variety of biological settings.[1] PD 098059 has been shown previously to inhibit the dephosphorylated form of mitogen-activated protein kinase kinase-1 (MAPKK1) and a mutant MAPKK1(S217E,S221E), which has low levels of constitutive activity (Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J., and Saltiel, A. R. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7686-7689). Here we report that PD 098059 does not inhibit Raf-activated MAPKK1 but that it prevents the activation of MAPKK1 by Raf or MEK kinase in vitro at concentrations (IC50 = 2-7 microM) similar to those concentrations that inhibit dephosphorylated MAPKK1 or MAPKK1(S217E,S221E). PD 098059 inhibited the activation of MAPKK2 by Raf with a much higher IC50 value (50 microM) and did not inhibit the phosphorylation of other Raf or MEK kinase substrates, indicating that it exerts its effect by binding to the inactive form of MAPKK1. PD 098059 also acts as a specific inhibitor of the activation of MAPKK in Swiss 3T3 cells, suppressing by 80-90% its activation by a variety of agonists. The high degree of specificity of PD 098059 in vitro and in vivo is indicated by its failure to inhibit 18 protein Ser/Thr kinases (including two other MAPKK homologues) in vitro by its failure to inhibit the in vivo activation of MAPKK and MAP kinase homologues that participate in stress and interleukin-1-stimulated kinase cascades in KB and PC12 cells, and by lack of inhibition of the activation of p70 S6 kinase by insulin or epidermal growth factor in Swiss 3T3 cells. PD 098059 (50 microM) inhibited the activation of p42MAPK and isoforms of MAP kinase-activated protein kinase-1 in Swiss 3T3 cells, but the extent of inhibition depended on how potently c-Raf and MAPKK were activated by any particular agonist and demonstrated the enormous amplification potential of this kinase cascade. PD 098059 not only failed to inhibit the activation of Raf by platelet-derived growth factor, serum, insulin, and phorbol esters in Swiss 3T3 cells but actually enhanced Raf activity. The rate of activation of Raf by platelet-derived growth factor was increased 3-fold, and the subsequent inactivation that occurred after 10 min was prevented. These results indicate that the activation of Raf is suppressed and that its inactivation is accelerated by a downstream component(s) of the MAP kinase pathway.[2] The mitogen-activated protein kinase (MAP kinase) pathway is thought to play an important role in the actions of neurotrophins. A small molecule inhibitor of the upstream kinase activator of MAP kinase, MAP kinase kinase (MEK) was examined for its effect on the cellular action of nerve growth factor (NGF) in PC-12 pheochromocytoma cells. PD98059 selectively blocks the activity of MEK, inhibiting both the phosphorylation and activation of MAP kinases in vitro. Pretreatment of PC-12 cells with the compound completely blocked the 4-fold increase in MAP kinase activity produced by NGF. Half-maximal inhibition was observed at 2 microM PD98059, with maximal effects at 10-100 microM. The tyrosine phosphorylation of immunoprecipitated MAP kinase was also completely blocked by the compound. In contrast, the compound was without effect on NGF-dependent tyrosine phosphorylation of the pp140trk receptor or its substrate Shc and did not block NGF-dependent activation of phosphatidylinositol 3'-kinase. However, PD98059 completely blocked NGF-induced neurite formation in these cells without altering cell viability. These data indicate that the MAP kinase pathway is absolutely required for NGF-induced neuronal differentiation in PC-12 cells.[3] Activation of the Raf/MEK/ERK pathway and inactivation of wild-type p53 by Mdm2 overexpression are frequent molecular events in acute myelogenous leukemia (AML). We investigated the interaction of Raf/MEK/ERK and p53 pathways after their simultaneous blockades using a selective small-molecule antagonist of Mdm2, Nutlin-3a, and a pharmacologic MEK-specific inhibitor, PD98059. We found that PD98059, which itself has minimal apoptogenic activity, acts synergistically with Nutlin-3a to induce apoptosis in wild-type p53 AML cell lines OCI-AML-3 and MOLM-13. Interestingly, PD98059 enhanced nuclear proapototic function of p53 in these cells. In accordance with the activation of transcription-dependent apoptosis, PD98059 treatment promoted the translocation of p53 from the cytoplasm to the nucleus in OCI-AML-3 cells, in which p53 primarily initiates transcription-independent apoptosis when cells are treated with Nutlin-3a alone. The critical role of p53 localization in cells with increased p53 levels was supported by enhanced apoptosis induction in cells cotreated with Nutlin-3a and the nuclear export inhibitor leptomycin B. PD98059 prevented p53-mediated induction of p21 at the transcriptional level. The repressed expression of antiapototic p21 also seemed to contribute to synergism between PD98059 and Nutlin-3a because (a) the synergistic apoptogenic effect was preserved in G(1) cells, (b) p53-mediated induction of p21 was preferentially seen in G(1) cells, (c) PD98059 strongly antagonized p21 induction by Nutlin-3a, and (d) cells with high p21 levels were resistant to apoptosis. This is the first report showing that the Raf/MEK/ERK pathway regulates the subcellular localization of p53 and the relative contribution of transcription-dependent and transcription-independent pathways in p53-mediated apoptosis.[10] Anticancer drugs docetaxel and vinorelbine suppress cell growth by altering microtubule assembly and activating the proapoptotic signal pathway. Vinorelbine and docetaxel have been approved for treating several advanced cancers. However, their efficacy in the management of advanced hormone-refractory prostate cancer remains to be clarified. Microtubule damage by some anticancer drugs can activate the ERK survival pathway, which conversely compromises chemotherapeutic efficacy. We analyzed the effect of ERK inhibitors PD98059 and U0126 on vinorelbine- and docetaxel-induced cell growth suppression of androgen-independent prostate cancer cells. In androgen-independent C-81 LNCaP cells, inhibition of ERK by PD98059, but not U0126, plus docetaxel resulted in enhanced growth suppression by an additional 20% compared to the sum of each agent alone (p < 0.02). The combination treatment of docetaxel plus PD98059 also increased cellular apoptosis, which was in part due to the inactivation of Bcl-2 by increasing phosphorylated Bcl-2 by more than 6-fold and Bax expression by 3-fold over each agent alone. At these dosages, docetaxel alone caused only marginal phosphorylation of Bcl-2 (10%). Docetaxel plus U0126 had only 20% added effect on Bcl-2 phosphorylation compared to docetaxel alone. Nevertheless, both U0126 and PD98059 exhibited an enhanced effect on docetaxel-induced growth suppression in PC-3 cells. No enhanced effect was observed for vinorelbine plus PD98059 or U0126. Thus, the combination therapy of docetaxel plus PD98059 may represent a new anticancer strategy, requiring lower drug dosages compared to docetaxel monotherapy. This may lower the cytotoxicity and enhance tumor suppression in vivo. This finding of a combination effect could be of potential clinical importance in treating hormone-refractory prostate cancer.[9] Neuropathic pain treatment remains challenging due to ineffective therapy and resistance to opioid analgesia. Mitogen-activated protein kinase kinase (MAPKK) have been identified as the crucial regulators of pro- and antinociceptive factors. We used PD98059, an inhibitor of the MAPKK family members MEK1/2. The aim of study was to examine the influence of single and/or repeated PD98059 on nociception and opioid effectiveness in neuropathy. Moreover, we examined how PD98059 influences selected members of cellular pathways and cytokines. The PD98059 (2.5 mcg) was intrathecally preemptively administered before chronic constriction injury (CCI), and then once daily for 7 days. Additionally, at day 7 after CCI the PD98059-treated rats received a single injection of opioids. Using Western blot and qRT-PCR techniques in PD98059-treated rats we analyzed the mRNA and/or protein level of p38, ERK1/2, JNK, NF-kappaB, IL-1beta, IL-6, iNOS and IL-10 in the lumbar spinal cord. Our results indicate that PD98059 has an analgesic effects and potentiates morphine and/or buprenorphine analgesia. Parallel we observed that PD98059 inhibit upregulation of the CCI-elevated p38, ERK1/2, JNK and NF-kappaB protein levels. Moreover, PD98059 also prevented increase of pro- (IL-1beta, IL-6, and iNOS) but enhances anti-nociceptive (IL-10) factors. Summing up, PD98059 diminished pain and increased the effectiveness of opioids in neuropathy. The inhibition of MEKs might inactivate a variety of cell signaling pathways that are implicated in nociception. PLoS One. 2015 Oct 1;10(10):e0138583. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4591269/ |
| 分子式 |
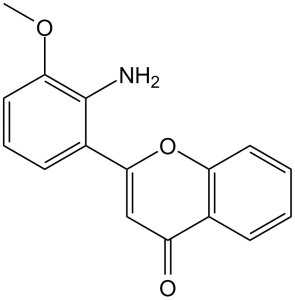
C16H13NO3
|
|---|---|
| 分子量 |
267.2793
|
| 精确质量 |
267.089
|
| 元素分析 |
C, 71.90; H, 4.90; N, 5.24; O, 17.96
|
| CAS号 |
167869-21-8
|
| 相关CAS号 |
167869-21-8
|
| PubChem CID |
4713
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
453.1±45.0 °C at 760 mmHg
|
| 熔点 |
170 °C
|
| 闪点 |
221.9±25.0 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.652
|
| LogP |
2.43
|
| tPSA |
65.46
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
407
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O1C2=C([H])C([H])=C([H])C([H])=C2C(C([H])=C1C1C([H])=C([H])C([H])=C(C=1N([H])[H])OC([H])([H])[H])=O
|
| InChi Key |
QFWCYNPOPKQOKV-UHFFFAOYSA-N
|
| InChi Code |
nChI=1S/C16H13NO3/c1-19-14-8-4-6-11(16(14)17)15-9-12(18)10-5-2-3-7-13(10)20-15/h2-9H,17H2,1H3
|
| 化学名 |
2-(2-amino-3-methoxyphenyl)chromen-4-one
|
| 别名 |
PD98059; PD 98059; PD098059; 2-(2-amino-3-methoxyphenyl)-4H-chromen-4-one; PD-98059; 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one; PD 98,059; 2-(2-amino-3-methoxyphenyl)chromen-4-one; PD-98059
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 33.3~46 mg/mL (124.7~172.1 mM)
Ethanol: Insoluble (<1 mg/mL) Water: Insoluble (<1 mg/mL) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (7.78 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O: 1mg/mL View More
配方 3 中的溶解度: 10 mg/mL (37.41 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7414 mL | 18.7070 mL | 37.4139 mL | |
| 5 mM | 0.7483 mL | 3.7414 mL | 7.4828 mL | |
| 10 mM | 0.3741 mL | 1.8707 mL | 3.7414 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of PD98059 on the development of neuropathic pain symptoms.
Effect of PD98059 on the mRNA and protein level of pro-inflammatory factors (IL-1beta, iNOS, IL-6 and IL-18) and anti-inflammatory factor (IL-10) in neuropathic pain.PLoS One.2015 Oct 1;10(10):e0138583. |
Effect of PD98059 on the p38, ERK1/2, JNK and NF-kappaB protein level in neuropathic pain.PLoS One.2015 Oct 1;10(10):e0138583. |
Effect of PD98059 on opioid analgesia in a naive and neuropathic rats. |