| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PI3Kδ (Ki = 1.6 nM); PI3Kα (Ki = 1.8 nM); PI3Kγ (Ki = 1.9 nM); PI3Kβ (Ki = 2.1 nM); mTOR (Ki = 16 nM)
|
|---|---|
| 体外研究 (In Vitro) |
PF-04691502 在生化测定中抑制重组 I 类 PI3K 和 mTOR,并防止野生型 PI3K γ、δ 或突变型 PI3Kα 引起的禽类成纤维细胞转化。 PF-04691502 抑制细胞增殖(IC(50) 为 179-313 nM)并分别降低 PIK3CA 突变型和 PTEN 缺失型癌细胞系中 AKT T308 和 AKT S473 的磷酸化。通过不依赖 PI3K 的营养刺激测定测定,PF-04691502 抑制细胞中 mTORC1 的活性,IC(50) 为 32 nM。它还可以防止 PI3K 和 mTOR 下游效应器的激活,例如 AKT、FKHRL1、PRAS40、p70S6K、4EBP1 和 S6RP。虽然 mTOR 抑制在暴露于 PF-04691502 后持续 24 至 48 小时,但短期暴露主要抑制 PI3K。除了上调 p27 Kip1 和下调 Rb 之外,PF-04691502 还会导致细胞周期 G(1) 停滞。 [1]
|
| 体内研究 (In Vivo) |
在 SKOV3(PIK3CA 突变)、U87(PTEN 缺失)以及吉非替尼和厄洛替尼耐药的非小细胞肺癌异种移植物中,观察到 PF-04691502 的抗肿瘤活性。 [1] 7 天后,PF-04691502 使肿瘤生长减少 72%。 FDG-PET 成像表明 PF-04691502 显着降低葡萄糖代谢。 PF-04691502 治疗后,PI3K/mTOR 通路活性的两种组织生物标志物 p-AKT (S473) 和 p-RPS6 (S240/244) 也受到严重抑制。 [2]
|
| 酶活实验 |
以下程序用于 ATP 竞争性抑制荧光偏振测定:在新鲜测定缓冲液(50 mM Hepes pH 7.4、150 mM NaCl、5 mM DTT、0.05% CHAPS)中制备 mPI3Kα 稀释溶液 (90 nM),并保存在冰上。酶反应含有 0.5 nM 小鼠 PI3Kα(从昆虫细胞中纯化的 p110α/p85α 复合物)、30 μM PIP2、PF-04691502(0、1、4 和 8 nM)、5 mM MgCl2 和 2 倍连续稀释的 ATP (0-800μM)。成品中二甲亚砜含量为2.5%。使用 ATP 启动反应,30 分钟后使用 10 mM EDTA 停止反应。在检测板中,将 15 uL 激酶反应混合物与 15 uL 检测器/探针混合物混合,其中在测定缓冲液中含有 480 nM GST-Grp1PH 结构域和 12 nM TAMRA 标记的荧光 PIP3。在 LJL Analyst HT 上读取板之前,将其摇动 3 分钟并孵育 35 至 40 分钟。
|
| 细胞实验 |
在含有 10% FBS 的生长培养基的 96 孔培养板中,以每孔 3,000 个细胞的密度接种 BT20、U87MG 和 SKOV3 细胞。过夜孵育后,将 DMSO(最终浓度为 0.1%)或连续稀释的化合物应用于细胞。添加刃天青浓度为 0.1 mg/mL。将板在 37 °C、5% CO2 中孵育三个小时。在 530 nm 处激发后,荧光信号被读取为 590 nm 处的发射。将荧光强度和药物浓度绘制在非线性曲线上以确定 IC50 值。
|
| 动物实验 |
Mice: Female nu/nu mice (6-8 weeks old) are used. To prepare for implantation, tumor cells are removed and then re-suspended in matrigel (1:1) and serum-free medium. The region of the back flank is subcutaneously implanted with SKOV3, U87MG, or NSCLC cells (2.5-4 106). When a tumor's size ranges from 100 to 200 mm3, treatment can begin. The daily oral administration of PF-04691502 contains 0.5% methylcellulose in water suspension. Every two to three days, tumor volumes and animal body weights are measured. Vernier calipers are used to measure and calculate tumor volume. Calculated tumor growth inhibition (TGI) percentage. Data are shown as mean±SE.
|
| 参考文献 | |
| 其他信息 |
PF-04691502 is pI3K/mTOR Kinase Inhibitor PF-04691502 is an agent targeting the phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in the PI3K/mTOR signaling pathway, with potential antineoplastic activity.
PF-04691502 has been used in trials studying the treatment of Cancer, Breast Neoplasms, Early Breast Cancer (Phase 2), and Advanced Breast Cancer (Phase 1b). PI3K/mTOR Kinase Inhibitor PF-04691502 is an agent targeting the phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in the PI3K/mTOR signaling pathway, with potential antineoplastic activity. PI3K/mTOR kinase inhibitor PF-04691502 inhibits both PI3K and mTOR kinases, which may result in apoptosis and growth inhibition of cancer cells overexpressing PI3K/mTOR. Activation of the PI3K/mTOR pathway promotes cell growth, survival, and resistance to chemotherapy and radiotherapy; mTOR, a serine/threonine kinase downstream of PI3K, may also be activated independent of PI3K. Deregulation of the phosphoinositide 3-kinase (PI3K) signaling pathway such as by PTEN loss or PIK3CA mutation occurs frequently in human cancer and contributes to resistance to antitumor therapies. Inhibition of key signaling proteins in the pathway therefore represents a valuable targeting strategy for diverse cancers. PF-04691502 is an ATP-competitive PI3K/mTOR dual inhibitor, which potently inhibited recombinant class I PI3K and mTOR in biochemical assays and suppressed transformation of avian fibroblasts mediated by wild-type PI3K γ, δ, or mutant PI3Kα. In PIK3CA-mutant and PTEN-deleted cancer cell lines, PF-04691502 reduced phosphorylation of AKT T308 and AKT S473 (IC(50) of 7.5-47 nmol/L and 3.8-20 nmol/L, respectively) and inhibited cell proliferation (IC(50) of 179-313 nmol/L). PF-04691502 inhibited mTORC1 activity in cells as measured by PI3K-independent nutrient stimulated assay, with an IC(50) of 32 nmol/L and inhibited the activation of PI3K and mTOR downstream effectors including AKT, FKHRL1, PRAS40, p70S6K, 4EBP1, and S6RP. Short-term exposure to PF-04691502 predominantly inhibited PI3K, whereas mTOR inhibition persisted for 24 to 48 hours. PF-04691502 induced cell cycle G(1) arrest, concomitant with upregulation of p27 Kip1 and reduction of Rb. Antitumor activity was observed in U87 (PTEN null), SKOV3 (PIK3CA mutation), and gefitinib- and erlotinib-resistant non-small cell lung carcinoma xenografts. In summary, PF-04691502 is a potent dual PI3K/mTOR inhibitor with broad antitumor activity. PF-04691502 has entered phase I clinical trials.[1] The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is commonly dysregulated in human cancer, making it an attractive target for novel anticancer therapeutics. We have used a mouse model of ovarian cancer generated by Kras(G12D) activation and Pten deletion in the ovarian surface epithelium for the preclinical assessment of a novel PI3K/mTOR inhibitor PF-04691502. To enable higher throughput studies, we developed an orthotopic primary transplant model from these mice and evaluated therapeutic response to PF-04691502 using small-animal ultrasound and FDG-PET imaging. PF-04691502 inhibited tumor growth at 7 days by 72% ± 9. FDG-PET imaging revealed that PF-04691502 reduced glucose metabolism dramatically, suggesting FDG-PET may be exploited as an imaging biomarker of target inhibition by PF-04691502. Tissue biomarkers of PI3K/mTOR pathway activity, p-AKT (S473), and p-RPS6 (S240/244), were also dramatically inhibited following PF-04691502 treatment. However, as a single agent, PF-04691502 did not induce tumor regression and the long-term efficacy was limited, with tumor proliferation continuing in the presence of drug treatment. We hypothesized that tumor progression was because of concomitant activation of the mitogen-activated protein kinase pathway downstream of Kras(G12D) expression promoting cell survival and that the therapeutic effect of PF-04691502 would be enhanced by combinatory inhibition of MEK using PD-0325901. This combination induced striking tumor regression, apoptosis associated with upregulation of Bim and downregulation of Mcl-1, and greatly improved duration of survival. These data suggest that contemporaneous MEK inhibition enhances the cytotoxicity associated with abrogation of PI3K/mTOR signaling, converting tumor growth inhibition to tumor regression in a mouse model of ovarian cancer driven by PTEN loss and mutant K-Ras. [1] The role of PI3K and MAPK pathways in tumor initiation and progression is well established; hence, several inhibitors of these pathways are currently in different stages of clinical trials. Recent studies identified a PI3K/mTOR (PF-04691502) and a MEK inhibitor (PD-0325901) with strong potency and efficacy in different cell lines and tumor models. PD-0325901, however, showed adverse effects when administered at or above MTD (maximum tolerated dose) in the clinic. Here, we show in preclinical models that PD-0325901 at doses well below MTD (sub-MTD 1.5 mg/kg SID) is still a potent compound as single agent or in combination with PF-04691502. We first observed that PD-0325901 at 1.5 mg/kg SID and in combination with PF-04691502 (7.5 mg/kg; SID) significantly inhibited growth of H460 (carry Kras and PIK3CA mutations) orthotopic lung tumors. Additionally, we tested efficacy of PD-0325901 in Kras(G12D-LSL) conditional GEMMs (genetically engineered mouse models) which are a valuable tool in translational research to study tumor progression. Intranasal delivery of adenoviruses expressing Cre recombinase (Adeno-Cre) resulted in expression of mutant Kras leading to development of tumor lesions in lungs including adenomatous hyperplasia, large adenoma, and adenocarcinoma. Similar to H460 tumors, PD-0325901 as single agent or in combination with PF-04691502 significantly inhibited growth of tumor lesions in lungs in Kras(G12D-LSL) mice when treatment started at adenocarcinoma stage (at 14 weeks post-Adeno-Cre inhalation). In addition, immunohistochemistry showed inhibition of pS6 (phosphorylated ribosomal S6) in the treated animals particularly in the combination group providing a proof of mechanism for tumor growth inhibition. Finally, m-CT imaging in live Kras(G12D-LSL) mice showed reduction of tumor burdens in PD-0325901-treated animals at sub-MTD dose. In conclusion, our data suggest that PD-0325901 at doses below MTD is still a potent compound capable of tumor growth inhibition where Kras and/or PI3K are drivers of tumor growth and progression. [3] |
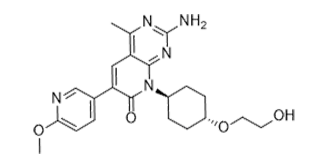
| 分子式 |
C22H27N5O4
|
|
|---|---|---|
| 分子量 |
425.48
|
|
| 精确质量 |
425.206
|
|
| 元素分析 |
C, 62.10; H, 6.40; N, 16.46; O, 15.04
|
|
| CAS号 |
1013101-36-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
25033539
|
|
| 外观&性状 |
Off-white to gray solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
682.5±65.0 °C at 760 mmHg
|
|
| 闪点 |
366.5±34.3 °C
|
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
|
| 折射率 |
1.646
|
|
| LogP |
1.43
|
|
| tPSA |
125.38
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
654
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O(C([H])([H])C([H])([H])O[H])C1([H])C([H])([H])C([H])([H])C([H])(C([H])([H])C1([H])[H])N1C(C(C2=C([H])N=C(C([H])=C2[H])OC([H])([H])[H])=C([H])C2=C(C([H])([H])[H])N=C(N([H])[H])N=C12)=O
|
|
| InChi Key |
XDLYKKIQACFMJG-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)
|
|
| 化学名 |
2-amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.88 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3503 mL | 11.7514 mL | 23.5029 mL | |
| 5 mM | 0.4701 mL | 2.3503 mL | 4.7006 mL | |
| 10 mM | 0.2350 mL | 1.1751 mL | 2.3503 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Kinross KM, Mol Cancer Ther, 2011, 10(8), 1440-1449 |
 |
 |