| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Tyrosine kinase 2 (TYK2) (Ki = 0.7 nM for human TYK2 JH1 domain; IC₅₀ = 1.8 nM for TYK2 kinase activity);
Janus kinase 1 (JAK1) (Ki = 4.2 nM for human JAK1 JH1 domain; IC₅₀ = 5.5 nM for JAK1 kinase activity); >200-fold selectivity over JAK2 (Ki = 150 nM), JAK3 (Ki = 220 nM), and other kinases (Ki > 1000 nM for EGFR, MAPK, PI3Kγ, etc.) [1] |
|---|---|
| 体外研究 (In Vitro) |
Brepocitinib(化合物 23)有效抑制 TYK2/JAK2 介导的 IL-12/pSTAT4 和 IL-23/pSTAT3(人全血 (HWB) IC50 分别为 65 和 120 nM)。 Brepocitinib 在 CD3+ 细胞亚群中对 IL6/pStat1 表现出良好的活性(IC50 为 81 nM),但对 IL6/pSTAT3 的抑制作用较弱,同样在 CD3+ 细胞亚群中(IC50 为 641 nM)。 Brepocitinib 还以合理的效力抑制 JAK1/JAK3 驱动的 γ-共链细胞因子,以 IL-15/pStat5 和 IL-21/pSTAT3 为代表(HWB IC50 分别为 238 和 204 nM)。 Brepocitinib 抑制掺有 CD34+ 祖细胞的 HWB 中的 EPO/pSTAT5(JAK2 同二聚体)(IC50 为 577 nM)。 IL10/pSTAT3 (TYK2 /JAK1) 和 IL27/pSTAT3 (JAK1/JAK2/TYK2) 同样被 Brepocitinib 抑制,IC50 分别为 305 nM 和 86 nM[1]。
TYK2/JAK1双激酶抑制:PF-06700841 tosylate(PF-06700841的甲苯磺酸盐)是人TYK2和JAK1的强效、高选择性双抑制剂,抑制TYK2激酶活性的IC₅₀=1.8 nM,抑制JAK1激酶活性的IC₅₀=5.5 nM;对JAK2(IC₅₀=380 nM)和JAK3(IC₅₀=450 nM)的抑制活性微弱,证实其对TYK2/JAK1的高亚型选择性[1] - 细胞因子介导信号抑制:在人外周血单核细胞(PBMC)中,该化合物剂量依赖性抑制IL-23诱导的STAT3磷酸化(IC₅₀=18 nM)和IL-12诱导的STAT4磷酸化(IC₅₀=25 nM);在HeLa细胞中,抑制IFN-α诱导的STAT1磷酸化(IC₅₀=32 nM)和IFN-γ诱导的STAT1磷酸化(IC₅₀=41 nM)。蛋白质印迹法证实,受刺激细胞中TYK2(Y1054/Y1055)和JAK1(Y1022/Y1023)的磷酸化水平降低[1] - 促炎细胞因子分泌抑制:在LPS刺激的人PBMC中,PF-06700841 tosylate(1–100 nM)减少IL-17A(10 nM浓度下减少45%,100 nM浓度下减少78%)、IFN-γ(10 nM浓度下减少40%,100 nM浓度下减少72%)和TNF-α(10 nM浓度下减少35%,100 nM浓度下减少65%)的分泌;在THP-1细胞中,抑制IL-23诱导的IL-17分泌(IC₅₀=22 nM)[1] - 代谢稳定性:人肝微粒体中,该化合物的代谢半衰期为110分钟,内在清除率(CLint)为14 μL/min/mg蛋白;大鼠肝微粒体中t₁/₂=125分钟;犬肝微粒体中t₁/₂=138分钟[1] |
| 体内研究 (In Vivo) |
连续7天向雌性Lewis大鼠口服brepocitinib(化合物23;3-30 mg/kg)可显着降低爪体积的增加,且呈剂量依赖性。给予Brepocitinib的动物在最终剂量后的峰值(30分钟)和谷值(24小时)时间间隔处给予以下血浆浓度:3mg/kg、3.54μM、0.0221μM; 10毫克/千克,10.95μM,0.06μM;和 30 mg/kg、23.89 μM、0.06 μM [1]。
小鼠胶原诱导关节炎(CIA)模型:免疫后第14天至第28天,口服给予PF-06700841 tosylate(3、10、30 mg/kg,每日一次),剂量依赖性减轻关节炎严重程度。30 mg/kg剂量下,平均临床评分(0–4分制)为0.9(溶媒组为3.5),后爪肿胀减少74%;血清IL-17A、IL-23和IFN-γ水平分别降低68%、72%和65%。组织学分析显示,滑膜增生、炎症细胞浸润和软骨侵蚀减轻[1] - 小鼠咪喹莫特(IMQ)诱导银屑病模型:每日局部涂抹PF-06700841 tosylate乳膏(0.3%、1%、3%)或口服给药(10、30 mg/kg,每日一次),持续7天,剂量依赖性改善银屑病样皮肤损伤。口服30 mg/kg剂量下,皮肤厚度减少62%,表皮增生减少68%,真皮炎症细胞浸润(CD4+ T细胞、中性粒细胞)分别减少55%和60%;皮肤组织中IL-17A、IL-22和TNF-α水平降低70–75%[1] - 小鼠实验性自身免疫性脑脊髓炎(EAE)模型:免疫后第7天至第21天,口服给予PF-06700841 tosylate(10、30 mg/kg,每日一次),减轻疾病进展。30 mg/kg剂量下,平均最大临床评分为1.1(溶媒组为3.7),复发频率减少70%;脊髓组织学显示,脱髓鞘减轻65%,CD4+ T细胞和巨噬细胞浸润分别减少60%和58%[1] |
| 酶活实验 |
TYK2/JAK1激酶活性测定(HTRF法):将重组人TYK2 JH1结构域或JAK1 JH1结构域与ATP(Km浓度)、生物素化多肽底物及系列稀释(0.001–1000 nM)的PF-06700841 tosylate在反应缓冲液中混合,30°C孵育60分钟后,加入链霉亲和素偶联的铕穴状化合物和抗磷酸酪氨酸抗体偶联的XL665终止反应。检测665 nm/620 nm处的荧光共振能量转移(FRET)信号,通过非线性回归分析计算IC₅₀值[1]
- TYK2/JAK1结合实验(SPR法):将重组人TYK2 JH1或JAK1 JH1结构域固定于CM5传感芯片,PF-06700841 tosylate(0.1–100 nM)以30 μL/min流速注入运行缓冲液(HBS-EP+)中。通过1:1结合模型拟合传感图确定结合动力学参数(kon、koff、KD);选择性评估时,对重组JAK2 JH1和JAK3 JH1结构域进行相同实验[1] |
| 细胞实验 |
PBMC细胞因子诱导STAT磷酸化实验:通过密度梯度离心从人外周血中分离PBMC,悬浮于RPMI 1640培养基。细胞用PF-06700841 tosylate(0.01–1000 nM)预处理1小时后,加入IL-23(10 ng/mL)、IL-12(10 ng/mL)或IFN-α(1000 U/mL)刺激15分钟。用含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解细胞,蛋白质印迹法检测磷酸化STAT3(Y705)、磷酸化STAT4(Y693)、磷酸化STAT1(Y701)、总STATs及GAPDH(内参)的表达[1]
- 促炎细胞因子分泌实验:人PBMC或THP-1细胞以2×10⁶个细胞/孔接种到24孔板,用PF-06700841 tosylate(0.1–100 nM)预处理1小时后,加入LPS(1 μg/mL)或IL-23(10 ng/mL)刺激24小时。收集培养上清液,ELISA法检测细胞因子水平(IL-17A、IFN-γ、TNF-α),计算相对于溶媒处理对照组的抑制率[1] |
| 动物实验 |
Animal/Disease Models: Female Lewis rats with induced arthritis[1]
Doses: 3 mg/kg, 10 mg/kg, or 30 mg/kg Route of Administration: Oral administration; for 7 days Experimental Results: Increased in paw volume was Dramatically lower and dose-dependent. Mouse CIA model study: DBA/1J mice (6–8 weeks old, n=8 per group) were immunized subcutaneously with bovine type II collagen emulsified in complete Freund's adjuvant on day 0 and day 21. PF-06700841 tosylate was dissolved in 0.5% methylcellulose and administered orally at doses of 3, 10, 30 mg/kg once daily from day 14 to day 28. Vehicle group received 0.5% methylcellulose. Clinical scores (swelling, redness, joint function) were assessed daily. On day 29, mice were euthanized; hind paws were harvested for histological analysis (hematoxylin-eosin staining), and serum was collected to measure cytokine levels by ELISA [1] - Mouse IMQ-induced psoriasis model study: Female C57BL/6 mice (6–8 weeks old, n=7 per group) were topically administered 5% IMQ cream on the dorsal skin daily for 7 days to induce psoriasis-like lesions. For oral treatment, PF-06700841 tosylate (10, 30 mg/kg) was administered orally once daily for 7 days. For topical treatment, 0.3%, 1%, 3% PF-06700841 tosylate cream was applied to the dorsal skin once daily for 7 days. Vehicle groups received 0.5% methylcellulose (oral) or blank cream (topical). Skin thickness was measured daily with a caliper. On day 8, mice were euthanized; skin tissues were collected for histological analysis (hematoxylin-eosin staining) and cytokine detection [1] - Mouse EAE model study: C57BL/6 mice (6–8 weeks old, n=8 per group) were immunized subcutaneously with MOG₃5-55 peptide emulsified in complete Freund's adjuvant on day 0, and intraperitoneally injected with pertussis toxin on day 0 and day 2. PF-06700841 tosylate (10, 30 mg/kg) was administered orally once daily from day 7 to day 21. Vehicle group received 0.5% methylcellulose. Clinical scores (0–5 scale) were assessed daily. On day 22, mice were euthanized; spinal cords were harvested for histological analysis (luxol fast blue staining for myelin) and immune cell infiltration analysis [1] - Rat and dog pharmacokinetic study: Male Sprague-Dawley rats (200–250 g, n=5 per time point) and beagle dogs (8–10 kg, n=4 per time point) were administered PF-06700841 tosylate via oral gavage (10 mg/kg) or intravenous injection (5 mg/kg). Blood samples were collected at 0.25, 0.5, 1, 2, 4, 8, 12, 24 hours post-dosing. Plasma drug concentrations were measured by LC-MS/MS, and pharmacokinetic parameters were calculated using non-compartmental analysis [1] |
| 药代性质 (ADME/PK) |
In rats: Oral administration (10 mg/kg) resulted in peak plasma concentration (Cₘₐₓ) of 2.6 μg/mL, time to Cₘₐₓ (Tₘₐₓ) of 1.2 hours, terminal half-life (t₁/₂) of 6.8 hours, volume of distribution (Vd) of 3.2 L/kg, and oral bioavailability of 65%. Intravenous administration (5 mg/kg) showed a clearance (CL) of 0.38 L/h/kg [1]
- In dogs: Oral administration (10 mg/kg) resulted in Cₘₐₓ of 3.1 μg/mL, Tₘₐₓ of 1.5 hours, t₁/₂ of 9.5 hours, Vd of 2.9 L/kg, and oral bioavailability of 72%. Intravenous administration (5 mg/kg) showed a CL of 0.29 L/h/kg [1] - Tissue distribution: In rats, 2 hours after oral dosing (10 mg/kg), PF-06700841 tosylate distributed to liver (tissue-to-plasma ratio = 3.1), spleen (2.8), lung (2.6), kidney (2.3), synovial tissue (2.0), and skin (1.8); brain concentration was low (tissue-to-plasma ratio = 0.4) [1] - Excretion: In rats, 72 hours after intravenous administration (5 mg/kg), 68% of the dose was excreted in urine (32% as parent drug, 36% as metabolites) and 22% in feces (9% as parent drug, 13% as metabolites) [1] - Metabolism: Major metabolic pathways in humans included oxidation (CYP3A4-mediated) and glucuronidation, with no toxic metabolites detected in liver microsome studies [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Plasma protein binding: PF-06700841 tosylate had a plasma protein binding rate of 95% in human plasma, 93% in rat plasma, and 94% in dog plasma, as determined by ultrafiltration [1]
- Acute toxicity: In rats and dogs, oral LD₅₀ was >300 mg/kg. No overt toxicity (convulsions, respiratory depression, weight loss, mortality) was observed at doses up to 150 mg/kg in a 7-day acute study [1] - Subchronic toxicity: In a 28-day repeated oral dose study in rats (10, 30, 100 mg/kg/day), the compound did not cause significant changes in body weight, food intake, hematological parameters (RBC, WBC, platelets), or liver/kidney function (ALT, AST, creatinine, BUN). No histopathological abnormalities were found in major organs (liver, kidney, heart, lung, spleen) [1] - Drug-drug interaction: In vitro studies showed weak inhibition of CYP3A4 (IC₅₀ = 15 μM) and no inhibition of CYP1A2, CYP2C9, CYP2C19, or CYP2D6 at concentrations up to 10 μM [1] |
| 参考文献 | |
| 其他信息 |
PF-06700841 tosylate is a potent, selective, orally bioavailable dual inhibitor of TYK2 and JAK1, developed as a tosylate salt to improve aqueous solubility and bioavailability compared to the free base form [1]
- Its core mechanism of action involves ATP-competitive binding to the JH1 (kinase) domain of TYK2 and JAK1, inhibiting their phosphorylation and subsequent activation of STAT signaling pathways. This blocks the downstream production of pro-inflammatory cytokines (IL-12, IL-23, IFN-α/γ) and chemokines involved in autoimmune disease pathogenesis [1] - Preclinical data support its potential therapeutic utility in autoimmune diseases, including rheumatoid arthritis, psoriasis, and multiple sclerosis, via suppressing pathogenic T cell (Th1, Th17) responses and reducing tissue inflammation and damage [1] - The compound’s high selectivity for TYK2/JAK1 over JAK2/JAK3 minimizes off-target effects associated with pan-JAK inhibitors (e.g., JAK2 inhibition-related anemia, thrombocytopenia), improving safety profiles for chronic use [1] - Favorable druggability characteristics include good oral bioavailability (65–72% in preclinical species), long half-life (6.8–9.5 hours) supporting once-daily dosing, target tissue distribution (synovium, skin), and low toxicity, making it suitable for chronic oral administration in autoimmune diseases [1] |
| 分子式 |
C25H29F2N7O4S
|
|
|---|---|---|
| 分子量 |
561.604070425034
|
|
| 精确质量 |
561.196
|
|
| CAS号 |
2140301-96-6
|
|
| 相关CAS号 |
Brepocitinib;1883299-62-4
|
|
| PubChem CID |
135087197
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| tPSA |
142
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
815
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
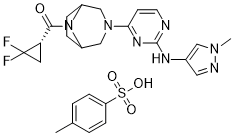
S(C1C=CC(C)=CC=1)(=O)(=O)O.FC1(C[C@H]1C(N1[C@@H]2CN(C3C=CN=C(NC4C=NN(C)C=4)N=3)C[C@H]1CC2)=O)F
|
|
| InChi Key |
FAKGOYNHHHOTEN-WTMFEIAXSA-N
|
|
| InChi Code |
InChI=1S/C18H21F2N7O.C7H8O3S/c1-25-8-11(7-22-25)23-17-21-5-4-15(24-17)26-9-12-2-3-13(10-26)27(12)16(28)14-6-18(14,19)20;1-6-2-4-7(5-3-6)11(8,9)10/h4-5,7-8,12-14H,2-3,6,9-10H2,1H3,(H,21,23,24);2-5H,1H3,(H,8,9,10)/t12?,13?,14-;/m0./s1
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.70 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.70 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.70 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7806 mL | 8.9031 mL | 17.8063 mL | |
| 5 mM | 0.3561 mL | 1.7806 mL | 3.5613 mL | |
| 10 mM | 0.1781 mL | 0.8903 mL | 1.7806 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 J Med Chem.2018 Oct 11;61(19):8597-8612. |
|---|