| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g | |||
| Other Sizes |
| 靶点 |
D2 receptor
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:吩噻嗪大部分在 10 位被二烷基氨基烷基取代,另外在 2 位被小基团取代,表现出有价值的活性,如精神安定、止吐、抗组胺、止痒、镇痛和抗蠕虫。 2-三氟甲基-10-(4-氨基丁基)phenothiazine 抑制 S. cerevisiae 菌株和 T. mentagrophites,MIC 分别为 0.4 μg/mL 和 1.5 μg/mL。 10-氨基甲酰基烷基吩噻嗪对革兰氏阳性枯草芽孢杆菌具有显着的活性,MIC 范围为 7.8 μg/mL–30 μg/mL。四环吩噻嗪(用萘醌环修饰)对金黄色葡萄球菌具有显着的抗菌活性,MIC50 为 12.5 μg/mL。带有丁烯接头的吩噻嗪比带有丙烯接头的吩噻嗪更有效,丙烯接头是 2-氯-10-氯乙基脲基丁基衍生物,针对 4 种白血病细胞系和 7 种结肠癌细胞系,其 GI50 分别为 1.4 μM 和 1.6 μM。 10-氨基(羟基)丙基吩噻嗪 (5 μM) 诱导人转化 WI38VA 细胞出现明显的细胞周期停滞 G2/M 期,随后在培养 2 天后发生细胞死亡。吩噻嗪类药物在体内经过广泛的代谢后被排出体外,主要是环羟基化、环磺氧化、N-去甲基化、N-氧化、硫酸化和葡萄糖醛酸结合。吩噻嗪对 α2 肾上腺素受体的结合亲和力比对多巴胺 D2 受体和 α1 肾上腺素受体的结合亲和力低得多。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
BECAUSE OF ITS LOW SOLUBILITY, RATE OF ITS ABSORPTION FROM GI TRACT IS DEPENDENT ON PARTICLE SIZE. MICRONIZED FORM OF DRUG IS ABSORBED RAPIDLY. ...ABOUT 30-50% OF ORAL DOSE PASSES THROUGH ALIMENTARY TRACT UNCHANGED. SOME PHENOTHIAZINE IS CONVERTED WITHIN GUT TO SOL DERIV...WHICH ARE ABSORBED INTO PORTAL VENOUS SYSTEM. ...DERIV ARE SECRETED IN URINE & ARE RESPONSIBLE FOR ITS RED COLOR WHEN EXPOSED TO AIR. THEY ALSO APPEAR IN BILE & IN MILK OF LACTATING ANIMALS. URINARY & FECAL EXCRETION OF PHENOTHIAZINE OR ITS DERIV ACCOUNTS FOR 80% OF ORAL DOSE IN SHEEP; FATE OF REMAINING 20% IS UNKNOWN. URINARY & FECAL EXCRETION OF PHENOTHIAZINE OR ITS DERIV ACCOUNTS FOR 80% OF ORAL DOSE IN SHEEP; FATE OF REMAINING 20% IS UNKNOWN. ABSORBED BY SKIN. ... Phenothiazine was readily absorbed from the alimentary tract, with the free drug and reddish oxidation products appearing in the urine. Metabolism / Metabolites SOME PHENOTHIAZINE IS CONVERTED WITHIN GUT TO SOL DERIV, MAINLY PHENOTHIAZINE SULFATE... YIELDS 3-HYDROXYPHENOTHIAZINE IN DOGS. /FROM TABLE/ THE ANTHELMINTIC, PHENOTHIAZINE, WAS OXIDIZED TO SULFOXIDE BY ENZYMES OF THE PROGLOTTIDS OF THE CESTODE, MONIEZIA EXPANSA & CYTOSOL OF INTESTINAL EPITHELIAL CELLS OF THE NEMATODE ASCARIS SUUM. ENZYMES IN THESE TISSUES ALSO DECR SULFOXIDES TO THIOETHERS IN ABSENCE OF O. RAT, MOUSE, & GERBIL EXCRETED THE MAJORITY OF PHENOTHIAZINE IN CONJUGATED FORM. THE RAT, MOUSE, & GERBIL PRODUCED LEUCOPHENOTHIAZONE SULFATE AS MAJOR METABOLITE, RELYING MORE ON C-OXIDATION PATHS THAN THE HAMSTER WHICH EXCRETED LARGE AMT OF PHENOTHIAZINE N-GLUCURONIDE. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
PREVIOUS DRENCHING WITH CARBON TETRACHLORIDE MAY ALSO SERVE TO INCR ITS TOXICITY. ANTIOXIDANTS, EG PHENOTHIAZINE, INHIBITED THE MUTAGENICITY OF BENZO(A)PYRENE & SOME OF ITS DERIVATIVES TOWARDS SALMONELLA TYPHIMURIUM STRAIN TA98; THIS INHIBITION WAS CONCN DEPENDENT. Non-Human Toxicity Values LD50 Rat oral 5000 mg/kg. |
||
| 参考文献 | |||
| 其他信息 |
Phenothiazine is a light green to steel-blue powder. Acquires a greenish-brown tint under exposure to sunlight. (NTP, 1992)
10H-phenothiazine is the 10H-tautomer of phenothiazine. It has a role as a plant metabolite, a radical scavenger and a ferroptosis inhibitor. It is a tautomer of a 4aH-phenothiazine, a 1H-phenothiazine and a 3H-phenothiazine. Phenothiazine (PTZ) is an organic thiazine compound. Phenothiazine has been reported in Mimosa pudica with data available. Phenothiazine is a class of agents exhibiting antiemetic, antipsychotic, antihistaminic, and anticholinergic activities. Phenothiazines antagonize the dopamine D2-receptor in the chemoreceptor trigger zone (CTZ) of the brain, potentially preventing chemotherapy-induced emesis. In addition, these agents have peripherally or centrally antagonistic activity against alpha adrenergic, serotonergic, histaminic, and muscarinic receptors. (NCI) See also: Promethazine (has subclass); Chlorpromazine (has subclass); Trifluoperazine (has subclass) ... View More ... Therapeutic Uses Antibiotics, Macrolide; Antiprotozoal Agents AT ONE TIME EMPLOYED IN HUMAN MEDICINE AS ANTHELMINTIC & URINARY ANTISEPTIC. MEDICATION (VET): ...EMPLOYED IN...VET MEDICINE FOR PINWORM, THREADWORM & ROUNDWORM INFESTATIONS. IT HAS ALSO BEEN USED AS URINARY ANTISEPTIC... MEDICATION (VET): IT IS STILL OF VALUE FOR TREATMENT OF HELMINTHIASIS IN SHEEP & OTHER DOMESTIC ANIMALS. For more Therapeutic Uses (Complete) data for PHENOTHIAZINE (8 total), please visit the HSDB record page. Drug Warnings VET: ...ANIMALS.../HAVE/ DIED AFTER RECEIVING...THERAPEUTIC DOSE, WHEREAS OTHERS HAVE SURVIVED MANY TIMES THIS AMT. ...VARIATION IN TOXICITY...ADEQUACY OR OTHERWISE OF DIET, & PARTICULARLY ITS PROTEIN CONTENT, IS IMPORTANT FACTOR. DEHYDRATION IS ANOTHER, MORTALITY IN LAMBS BEING HIGHEST UNDER DROUGHT CONDITIONS. SUSCEPTIBILITY TO TOXIC EFFECTS...CONSIDERED...GREATEST IN HORSE, LESS SO IN DOG & PIG, WHILE RUMINANTS & BIRDS APPEAR...RESISTANT. ...TOXIC EFFECTS... MORE FREQUENTLY IN CATTLE THAN IN SHEEP & GOATS. YOUNG & DEBILITATED ANIMALS...MORE SUSCEPTIBLE... DIGESTIVE DISTURBANCES...PROMOTE ABSORPTION... Use of the drug in weak animals, particularly those that are anemic and emaciated, is strictly contraindicated. Animals known to be constipated should not be treated with phenothiazine, since retention of the drug in the digestive tract and resulting absorption of greater than normal quantities is likely to lead to drug poisoning. Use of phenothiazine in pregnancy is contraindicated only during the last month of gestation. |
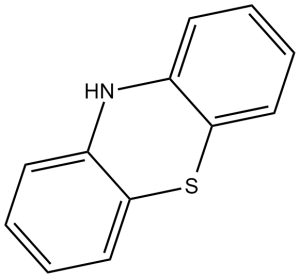
| 分子式 |
C12H9NS
|
|
|---|---|---|
| 分子量 |
199.27
|
|
| 精确质量 |
199.045
|
|
| 元素分析 |
C, 72.33; H, 4.55; N, 7.03; S, 16.09
|
|
| CAS号 |
92-84-2
|
|
| 相关CAS号 |
Phenothiazine-d8; 1219803-41-4
|
|
| PubChem CID |
7108
|
|
| 外观&性状 |
Off-white to light green solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
371.0±12.0 °C at 760 mmHg
|
|
| 熔点 |
184 °C
|
|
| 闪点 |
178.2±19.6 °C
|
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
|
| 折射率 |
1.675
|
|
| LogP |
4.15
|
|
| tPSA |
37.33
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
187
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S1C2C(=CC=CC=2)NC2C1=CC=CC=2
|
|
| InChi Key |
WJFKNYWRSNBZNX-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H9NS/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8,13H
|
|
| 化学名 |
10H-phenothiazine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (12.55 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (12.55 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0183 mL | 25.0916 mL | 50.1832 mL | |
| 5 mM | 1.0037 mL | 5.0183 mL | 10.0366 mL | |
| 10 mM | 0.5018 mL | 2.5092 mL | 5.0183 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。