| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| Other Sizes |
|
| 靶点 |
mGluR2/3
|
|---|---|
| 体外研究 (In Vitro) |
Pomaglumetad methionil (LY2140023) 是肽转运蛋白 1 (PEPT1) 的底物,具有很强的亲和力,Km 值约为 30 µM [2]。 LY2140023 表现出针对 [14C]Gly-Sar[2] 的活性,IC50 为 0.018 mM。
前药对PEPT1 [14C]Gly-Sar转运的抑制作用[2] 研究了Pomaglumetad methionil/LY2140023对PEPT1探针底物[14C]Gly-Sar (25 μM)在5 ~ 1000 μM浓度范围内的抑制作用。以LY2140023缺失时[14C]Gly-Sar的积累为阳性对照。在pcDNA3.1空载体转染的对照细胞中进行平行实验,测量各浓度下[14C]Gly-Sar的被动扩散,并从pept1转染的HeLa细胞中减去积累量。两次单独实验预估前药IC50值分别为0.023±0.09和0.013±0.07 mM,平均值为0.018 mM(表2)。 评价PEPT1对药前和活性片段的摄取。[2] 氨基酸前药Pomaglumetad methionil/LY2140023 (30 μM;前药)或活性药[14C]LY404039 (30 μM;在pH 6.0或7.5条件下,在短暂转染PEPT1的HeLa细胞中进行活性部分),以确定Pomaglumetad methionil/LY2140023或LY404039是否为PEPT1的底物(图2)。在转染PEPT1的HeLa细胞中,活性片段的积累水平与其被动积累相似,表明它没有被PEPT1转运。相反,如图2所示,前药摄取是一个质子依赖性和时间依赖性的过程,表明它是PEPT1底物。虽然通过pcDNA3.1空载体的摄取表明,前药的被动通透性略高于活性部分,但与PEPT1介导的ph依赖性转运相比仍然可以忽略不计。 已知PEPT1抑制剂对前药的IC50测定。[2] 利用二肽Gly-Sar作为抑制[14C]LY2140023摄取的阳性对照,测定了已知PEPT1底物对[14C]LY2140023/Pomaglumetad methionil(前药)转运的抑制电位。测定了几种已知PEPT1底物抑制10 μM [14C]LY2140023摄取的潜力;[14C]LY2140023摄取的两个独立实验的平均IC50值范围在0.46至25.90 mM之间(表2),其中伐昔洛韦是最有效的抑制剂,而左旋多巴是最不有效的抑制剂。[14C]Gly-Sar和前药的IC50效价排序相似,除了头孢氨苄是比卡托普利更有效的前药摄取抑制剂,而抑制Gly-Sar摄取的情况正好相反(表2)。LY2140023对Gly-Sar的抑制作用较强。 用体外数据评价临床意义。[2] 为了为pept1介导的相互作用的临床研究提供可能的抑制剂选择,我们比较了五种已上市药物(头孢丙塞、头孢氨苄、卡托普利、依那普利和伐昔洛韦)的体外IC50及其在胃肠道(GI)中的估计浓度(I2)。这些浓度(I2)是通过将推荐临床剂量除以250 ml(一杯水的体积)得到的(表3)。化合物的剂量取自1997年的《医师案头参考》。卡托普利(0.03 ~ 0.15)、头孢氨苄(0.18 ~ 0.37)和依那普利(0.008 ~ 0.03)的I2/IC50比值明显小于1(即估计GI浓度低于体外平均IC50值),而头孢地诺西(2.10 ~ 4.20)和伐昔洛韦(12.04 ~ 24.11)的I2/IC50比值大于1(即估计GI浓度大于体外平均IC50值),表明可能存在相互作用。使用80 mg剂量时,Pomaglumetad methionil/LY2140023的I2/IC50比伐昔洛韦高48.5。 |
| 体内研究 (In Vivo) |
Pomaglumetad methionil(LY2140023;口服;3-300 mg/kg;每天一次,持续 7 天)以剂量依赖性方式增加多巴胺代谢物高香草酸 (HVA) 和二羟基苯乙酸 (DOPAC) [1]。
理由:越来越多的证据表明,精神分裂症的原发性症状可能与中枢谷氨酸传递的改变有关。Pomaglumetad methionil/LY2140023一水合物是选择性mGlu(2/3)受体激动剂LY404039的蛋氨酸前药,目前正在评估用于治疗精神分裂症。 目的:评价Pomaglumetad methionil/LY2140023一水化合物在临床前和临床研究中的中心药理活性。 方法:评估雄性大鼠单次口服Pomaglumetad methionil/LY2140023一水化合物(前额叶皮质微转移物)、单次腹腔注射LY404039[脑脊液(CSF)]或LY2140023一水化合物(CSF)对神经递质/代谢物浓度的影响。一项16名健康受试者的临床研究评估了LY2140023一水40毫克,每日口服两次,连续14天对腰椎脑脊液的影响。 结果:大鼠研究:急性给药Pomaglumetad methionil/LY2140023一水化合物导致前额皮质细胞外二羟基苯基乙酸(DOPAC)和高香草酸(HVA)浓度显著增加,但不增加5-羟基吲哚乙酸(5-HIAA)。LY2140023一水给药7天后脑脊液中HVA浓度升高,而急性给药LY404039增加DOPAC、HVA和甲氧基羟基苯基乙二醇(MHPG)浓度,但不增加5-HIAA。临床研究:接受LY2140023一水合物治疗的受试者脑脊液中DOPAC、HVA、5-HIAA和MHPG显著升高,而安慰剂无此现象。 结论:Pomaglumetad methionil/LY2140023一水和/或LY404039给药可显著影响人和大鼠中枢神经系统多巴胺的转换,并显著影响血清素的转换。生物胺代谢物如DOPAC和HVA的测定可以作为LY2140023一水化合物和/或LY404039中心药效学活性的有用生物标志物。[1] 本研究共纳入24名健康受试者,其中男性8人,女性16人,平均年龄31.5岁(年龄范围19 ~ 66岁)。白种人12例(50.0%),非裔美国人11例(45.8%),亚裔1例(4.2%)。平均体重指数为26.6 kg/m2。 在24名受试者中,有20人完成了研究。4名受试者因以下原因停药:不符合资格标准(1名受试者在给药前停药)、AE(1)和违反方案23名受试者单独服用1000mg valacyclovir, 21名受试者单独服用80mg Pomaglumetad methionil/LY2140023, 20名受试者服用valacyclovir与LY2140023共给药。[2] |
| 酶活实验 |
前药及其活性部分的质子和时间依赖转运。[1]
转染24小时后测定PEPT1介导的[14C]LY2140023 (30 μM)和[14C]LY404039 (30 μM)的摄取。Pomaglumetad methionil/LY2140023和LY404039的时间依赖性转运使用上述pH为6.0或7.5制备的缓冲液进行。用空pcDNA3.1载体转染细胞,检测LY2140023和LY404039的被动扩散。细胞在室温下孵育1、2.5、5、7.5、10和15分钟,清洗、裂解,并按上述方法定量含量。 浓度依赖性摄取测定前药动力学参数。[1] [14C]LY2140023/Pomaglumetad methionil,范围为5 ~ 149 μM,在PEPT1转染的HeLa细胞中,室温培养2 ~ 3分钟,测定其浓度依赖性。平行实验中,用pcDNA3.1空载体转染HeLa细胞,得到各浓度下前药的平均被动扩散量,并减去PEPT1介导的摄取量。校正后的数据采用WinNonlin Professional软件,5.0.1或5.3版(Certara, l.p., St. Louis, MO)进行拟合。利用下式估算PEPT1介导的前药动力学参数: 抑制。[1] 以25 μM [14C] gy - sar (0.278 μCi/ml)为探针底物,测定前药(5 ~ 1000 μM)对[14C] gy - sar的摄取是否有抑制作用。细胞在含有25 μM [14C]Gly-Sar和不同浓度Pomaglumetad methionil/LY2140023的摄取缓冲液中室温孵育3分钟。在转染pcDNA3.1空载体的细胞中,通过平行实验确定背景,对摄取进行校正。如上所述进行细胞裂解和蛋白定量。 7种先前报道的PEPT1底物(Zhang et al., 2004)和Gly-Sar (PEPT1探针底物)也被评估,以确定它们对PEPT1介导的10 μM [14C]LY2140023 (0.193 μCi/ml)摄取的抑制潜力。上述两项单独的抑制研究使用ALA (0.1 ~ 5mm或0.25 ~ 5mm)、卡托普利(0.5 ~ 40mm)、头孢地诺西(0.3 ~ 10mm)、头孢氨苄(1 ~ 40mm)、依那普利(1 ~ 15mm或1 ~ 20mm)、左旋多巴(2.5 ~ 25mm)、Gly-Sar (0.1 ~ 5mm或0.25 ~ 5mm)和伐昔洛韦(0.1 ~ 5mm)进行。在这些浓度下,化合物的溶解度是用光纤光源检测的。对于左旋多巴,抑制浓度范围受溶解度限制。25 mM L-DOPA对[14C]LY2140023摄取的抑制作用不到50%,因此使用Gly-Sar在20 mM处的抑制作用作为PEPT1的完全抑制来估计IC50(假设在20 mM处完全抑制,因为Gly-Sar的IC50值为0.99 mM)。 研究药物测定[1] 采用经验证的液相色谱/串联质谱(LC/MS/MS)方法测定人血浆和脑脊液中Pomaglumetad methionil/LY2140023和LY404039浓度。这些方法按照既定的美国FDA生物分析方法验证和样品分析指南(CDER 2001)进行验证和实施。两种化合物在脑脊液中的定量下限和上限分别为0.5和100 ng/mL,血浆中的定量下限分别为1和100 ng/mL。CSF中Pomaglumetad methionil/LY2140023和LY404039的日间精度值分别为3.42%和8.53%的相对标准偏差(RSD), LY2140023的日间精度为- 5.12% ~ 1.06%的相对误差(RE), LY404039的日间精度为- 6.30% ~ - 2.71%的相对误差(RE)。在血浆中,LY2140023和LY404039的日间精度分别为7.13%和8.61% RSD,日间精度分别为- 2.69% ~ 5.71% RE和0% ~ 11.4% RE。样品与同位素内标混合,然后固相萃取分离分析物。在正离子模式下进行LC/MS/MS分析,并为每种分析物和内标选择特定的反应监测设置。分析运行包括校准标准品、质量控制样品和参与样品。发现超过定量上限的样品被稀释并重新分析。不符合校准曲线和质控样品精度先验接受标准的分析运行被拒绝,受影响的样品被重新分析。采用类似的LC/MS/MS技术测定大鼠血浆和脑脊液中LY404039的浓度。 |
| 动物实验 |
Animal/Disease Models: Male Fischer rat (approximately 250 g) [1]
Doses: 3 mg/kg, 10 mg/kg, and 300 mg/kg Route of Administration: Oral; one time/day for 7 days Experimental Results: Dose-dependent increase in dopamine Levels of the metabolites DOPAC and HVA. Preclinical methods [1] The vehicle for Pomaglumetad methionil/LY2140023 was distilled water; the vehicle for LY404039 was 0.01 N NaOH. Injection volumes were 5 mL/kg. Full descriptions of laboratory methods for each of the preclinical studies are provided in the ESM. Principles of laboratory animal care were followed in all preclinical studies. Rat acute dosing/in vivo microdialysis study [1] In brief, male Sprague–Dawley rats (approximately 300 g at the time of the study) were implanted with a guide cannula in the prefrontal cortex (PFC) (Paxinos and Watson 1986). Rats were allowed to acclimate overnight. Prior to the experiment, a concentric-type dialysis probe was inserted and the animals were maintained in a chamber allowing free movement. The inlet tube of the dialysis probe was perfused with artificial CSF at a final flow rate of 1.5 μL/min. Following equilibration, the fluid from the outlet was collected into a refrigerated fraction collector. Three baseline samples were collected at 30-min intervals. Pomaglumetad methionil/LY2140023 monohydrate (3, 10, or 30 mg/kg) or vehicle control was administered orally by gavage 20 min into the third sampling interval. Dialysate fractions were collected at 30-min intervals for the next 4.5 h. Rat CSF study [1] Pomaglumetad methionil/LY2140023 monohydrate was dosed orally once daily in male Fischer rats (Harlan Labs, Indianapolis, IN, approximately 250 g at the time of the study) for 7 days at 3, 10, and 300 mg/kg with sampling 3 h after last dose. LY404039 was dosed intraperitoneally in male Sprague–Dawley rats (Harlan Labs, approximately 250 g at the time of the study) 60 min before collection of CSF from the cisterna magna. Study design [1] This was an exploratory, randomized, subject-blind, placebo-controlled study including 16 healthy male subjects. The study (H8Y-FW-HBBF) was conducted. Subjects were randomized 3:1 to receive either 40 mg of Pomaglumetad methionil/LY2140023 monohydrate or placebo orally BID for 14 consecutive days. Dosing occurred at the investigative site, at approximately the same time each day for each subject. On day 1, a baseline lumbar puncture (LP) was performed in the morning immediately prior to the administration of the first dose of study drug. A blood sample for the determination of LY2140023 and LY404039 concentrations was collected on day 14 approximately 2 h after the last dose. The second CSF sample was collected by LP on the morning of day 15, approximately 10–12 h post-dose. A safety follow-up visit occurred 2 to 14 days after day 15. The study was terminated before completion for reasons unrelated to the study protocol. Therefore, the number of samples available for assay was smaller than expected because of the early termination. As such, a narrower panel of metabolites was assayed than originally intended. Study drug [1] All treatments were administered orally as solutions with 200 mL of room temperature water. Pomaglumetad methionil/LY2140023 monohydrate was reconstituted in a 0.42% sodium bicarbonate solution (8 mg/mL). Placebo treatment was given as 5 mL of 0.42% sodium bicarbonate solution. Clinical Study [2] The study was conducted as a three-period, fixed-sequence design in which subjects received a single dose of 1000 mg valacyclovir (Period 1), a single dose of 80 mg of Pomaglumetad methionil/LY2140023 (Period 2), and then a dose of 80 mg of LY2140023 coadministered with 1000 mg valacyclovir (Period 3); all periods were separated by a 5- to 10-day washout. Serial blood samples were collected for assessment of valacyclovir, acyclovir (refer to Ganapathy et al., 1998 for structure of valacyclovir and acyclovir), LY2140023, and LY404039 pharmacokinetics (PK). Urine was collected from 0 to 6, 6 to 12, and 12 to 24 hours postdose for analysis of valacyclovir, acyclovir, and/or the active moiety in the urine. Safety was assessed by collection of adverse events, clinical laboratory evaluations, electrocardiograms, and neurologic examinations. Eligible subjects were comprised of healthy men and women between 18 and 65 years of age, inclusive, with a body mass index between 19 and 32 kg/m2, inclusive. Subjects unable to cease use of xanthines, cigarettes, or over-the-counter or prescription medication for the duration of the trial were excluded. All subjects signed written informed consent before participation in the study. A sufficient number of subjects were enrolled to obtain 18 subjects to complete the study. This sample size was to provide at least 90% power to show the inclusion of the 90% confidence intervals (CI) of the ratio of area under the curve (AUC) geometric means between the test (LY2140023 + valacyclovir) and reference (LY2140023 alone) fall within the interval (0.80, 1.25). Bioanalysis. [2] Plasma samples were analyzed for valacyclovir, acyclovir, Pomaglumetad methionil/LY2140023 (prodrug), and LY404039 (active moiety) using validated turbo ion spray liquid chromatography/tandem mass spectrometric methods. For the prodrug and active moiety, the lower limit of quantification (LLQ) was 0.25 ng/ml and the upper limit of quantification (ULQ) was 100 ng/ml for both analytes (Annes et al., 2015). For valacyclovir and acyclovir, the LLQ was 100 ng/ml and the ULQ was 1000 ng/ml for both analytes. Urine samples were analyzed for valacyclovir, acyclovir, and/or active moiety using a validated liquid chromatography/tandem mass spectrometric method. No analysis of the prodrug was performed because previous studies have shown that it is not excreted in the urine. For the active moiety, the LLQ was 50 ng/ml and the ULQ was 5000 ng/ml. For valacyclovir and acyclovir, the LLQ was 100 ng/ml and the ULQ was 20,000 ng/ml. For all bioanalytical methods, samples above the limit of quantification were diluted and reanalyzed to yield results within the calibrated range. Pharmacokinetic Analyses. [2] Plasma concentration-time data for valacyclovir, acyclovir, Pomaglumetad methionil/LY2140023, and LY404039 were analyzed by standard noncompartmental methods of analysis using WinNonlin Version 5.3. Actual sampling times were used in the analyses with the exception of predose times, which were set to 0 hour. Area under the curve (AUC) values were determined using log-linear trapezoid methods. When calculating CL/F and Vz/F for the active moiety, the dose of the prodrug was adjusted based on the molar ratio of active moiety to prodrug (0.64). Urine concentration and volume data were measured for LY404039, valacyclovir, and acyclovir. Amounts excreted over each collection interval were summed to determine the cumulative amount excreted over the 24-hour collection interval [Ae(0–24)]. The fraction of the dose excreted (fe) was also determined. For the active moiety dose, the 0.64 correction factor was used as described previously. Similarly, for acyclovir, the valacyclovir dose was adjusted based on the molar ratio of acyclovir to valacyclovir (0.694). Apparent renal clearance was estimated using the cumulative amount excreted up to the last collection interval and plasma AUC(0–24). Although PK parameters were determined for all subjects with concentration-time data, if vomiting occurred within 5 hours postdose the concentration-time data and PK parameters from that dosing period were not included in any data summaries or statistical analysis. Only one subject (in Period 2) had PK data excluded because of vomiting. The primary PK parameters (Cmax and AUC) for Pomaglumetad methionil/LY2140023, LY404039, valacyclovir, and acyclovir were compared when the prodrug and valacyclovir were administered alone and in combination. AUC(0–∞) was used for all analytes except valacyclovir where AUC(0–3) was assessed. Parameters were compared using linear mixed effect model where treatment (80 mg of LY2140023 administered alone, 1000 mg valacyclovir administered alone, and 80 mg of LY2140023 coadministered with 1000 mg valacyclovir) was included as a fixed factor, and subject was a random factor. The parameters were log transformed before analysis. The least squares means (LSM) for each treatment and the 90% confidence intervals (CI) for the difference in means between test and reference treatment groups were estimated from the model and back transformed from the log scale to provide estimates of the geometric means and 90% CIs for the ratio of geometric means. The analysis of tmax was based on a nonparametric method. Medians and range for treatments and the P value computed for comparison of median values using Wilcoxon signed rank test are presented. Safety. [2] There were no serious adverse events (AEs) in this study. One subject discontinued from the study after experiencing a mild AE of urticaria that occurred approximately 4 hours after receiving 1000 mg of valacyclovir alone. Most AEs were mild or moderate; one severe AE of headache occurred after valacyclovir alone. The most common AEs after prodrug alone were nausea, dizziness, somnolence, and headache. The AE profile for Pomaglumetad methionil/LY2140023 coadministered with valacyclovir was similar to LY2140023 alone. |
| 药代性质 (ADME/PK) |
Pharmacokinetic evaluations [1]
Mean CSF concentrations at approximately 10–12 h post-dose were 1.93 and 4.93 ng/mL for Pomaglumetad methionil/LY2140023 and LY404039, respectively, and were quantifiable for all subjects. Pharmacokinetics. [2] After administration of the prodrug, the active moiety was formed rapidly and was present at the first sampling time in 18 of 21 subjects, which is consistent with previous clinical studies. Mean PK parameters (Table 4) and profiles (Fig. 4A) were similar after dosing of Pomaglumetad methionil/LY2140023 (the prodrug) alone and when coadministered with valacyclovir. Ratios of LSM for Cmax and AUC resulted in ratios that were close to 1 with confidence intervals contained within the 0.80 to 1.25 range (Table 5). Similarly, the plasma PK parameters (Table 4) and profiles (Fig. 4B) for LY404039 (the active moiety) were similar after dosing of the prodrug alone and when coadministered with valacyclovir. Urinary excretion of the active moiety was also similar for the prodrug alone and with valacyclovir (Table 4), as measured by the fraction excreted (fe; 0.651 and 0.595, respectively) and renal clearance (CLr; 12.8 and 12.2 l/h, respectively). Ratios of LSM for Cmax and AUC were also close to 1, and the CIs were contained within 0.80 and 1.25 (Table 5). The tmax analysis for prodrug and active moiety showed no significant differences observed for tmax (median of paired differences was 0.00 hour for prodrug and −0.07 hour for active moiety; Supplemental Table 1). The valacyclovir plasma concentrations are limited and typically only measurable for 3 or 4 hours postdose (Phan et al., 2003), because conversion from valacyclovir (prodrug) to acyclovir (active metabolite) is rapid and efficient. Mean plasma profiles of valacyclovir were similar whether administered alone or with prodrug (Fig. 5A). As shown in Table 6, the valacyclovir plasma PK parameters and renal clearance were similar when administered alone or with the prodrug. Ratios of LSM for Cmax and AUC were near 1 with CIs contained within the 0.80 to 1.25 range (Table 5), with the exception of the lower bound of the 90% CI for AUC(0–3), which was 0.71. After administration of the prodrug valacyclovir, the active metabolite acyclovir was formed rapidly. The acyclovir plasma PK and renal clearance were similar when administered alone or with the prodrug (Table 6). Similarly, the plasma profiles for acyclovir were similar with or without administration of the prodrug (Fig. 5B). The ratios of LSM for AUC and Cmax were close to 1, and the 90% CI were within the 0.80 to 1.25 range for acyclovir (Table 5). The tmax analysis for valacyclovir and acyclovir showed no differences observed for tmax (median of paired differences was 0.00 hour for valacyclovir and 0.00 hour for acyclovir; Supplemental Table 1). |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety [1]
No deaths or other serious adverse events occurred during this study; however, three subjects (two placebo- and one LY-treated) were withdrawn from the study by the investigator due to post-dural puncture headache (PDPH). The most common treatment-emergent adverse events possibly related to study treatment were nausea, vomiting, dizziness, and fatigue and were generally mild to moderate in severity (Table 4). Seven subjects experienced procedural complications involving PDPH. Of these, three (two placebo- and one Pomaglumetad methionil/LY2140023-treated) were withdrawn from the study by the investigator due to PDPH. The occurrences of PDPH were resolved completely with conservative management, and no epidural patches were performed for the treatment of PDPH. Safety of Pomaglumetad methionil/LY2140023 in the clinical study [1] LY2140023 monohydrate was generally well tolerated, with no clinically significant safety or tolerability issues related to LY2140023 noted in this study. Adverse events reported as possibly drug-related were similar to those observed in previous clinical studies with LY2140023 monohydrate. Procedure-related complications like PDPH can occur with lumbar puncture. Overall, the safety and tolerability of the LP procedures used in this study appeared comparable to prior experience. |
| 参考文献 |
|
| 其他信息 |
Effects of Pomaglumetad methionil/LY2140023 monohydrate and LY404039 on biogenic amine neurotransmitters and metabolites [1]
The present report shows that the mGlu2/3 agonist produg LY2140023 and/or LY404039 increased the CNS turnover of, in particular, the neurotransmitter DA as evidenced by the elevated levels of the metabolites DOPAC and HVA in rat brain dialysates and both rat and human CSF samples. These changes also appear to correlate with the plasma/CSF drug concentrations of LY404039. Thus, the CSF levels of these metabolites may serve as useful translational markers of LY2140023 and/or LY404039 central pharmacodynamic activity. In the preclinical studies described herein, LY404039 and the prodrug LY2140023 both increased biogenic amine turnover in the CNS of the rat. Thus, LY2140023 monohydrate administration produced dose-dependent increases in the extracellular levels of both DOPAC and HVA (Fig. 1) in vivo in the prefrontal cortical microdialysates with no major effects on the levels of 5-HIAA. These changes were in line with recently published rat microdialysis data for LY404039 where increased levels of extracellular DA were observed in the prefrontal cortex (Rorick-Kehn et al. 2007); that study also found increased levels of DOPAC and HVA in brain tissues postmortem. In the present study, we found similar effects in the rat CSF where single doses of LY404039 produced dose-dependent increases in the levels of DOPAC and HVA, and also the NE metabolite MHPG. After dosing with the prodrug LY2140023 monohydrate for 7 days, a similar trend was observed, but the effect was less pronounced with statistically significant elevations in HVA levels observed at 10 mg/kg. Importantly, the observed effects of LY2140023 and LY404039 on biogenic amine turnover in rat microdialysate and CSF were within the range of that which demonstrated efficacy in a rat phencyclidine model of schizophrenia (Patil et al. 2007b). In the clinical study reported herein, LY2140023 increased human CSF concentrations of DOPAC and HVA, as well as the serotonin metabolite 5-HIAA. The interpretation of lumbar concentrations of neurotransmitters and their metabolites is known to be limited by factors such as the craniocaudal gradient. However, in combination with data from the preclinical studies and the observed drug concentration effects both preclinically and clinically, these observations suggest that LY2140023 monohydrate administration also increases central dopaminergic and serotonergic turnover in humans. While significant increases in the concentrations of the NE metabolite MHPG were also observed, the lack of a statistically significant increase in DHPG concentrations in CSF precludes a clear conclusion regarding the effect of LY2140023 on central NE turnover. It is possible that the study was not sufficiently powered to adequately define the effects on noradrenergic turnover based on measurements of both NE metabolites. The observed effects of LY2140023 monohydrate on human CSF concentrations of biogenic amine metabolites occurred at a dose previously shown to be clinically efficacious in patients with schizophrenia (Patil et al. 2007b); therefore, the effects of LY2140023 monohydrate on biogenic amine metabolites in CSF were observed at a therapeutically relevant dose. In the present study, there were no significant changes in the concentrations of the biogenic amine metabolites assessed in the CSF of the placebo-treated subjects; however, due to the limited number of placebo-treated subjects in the present study, a definitive comparison between the active compound and placebo groups cannot be made. Nevertheless, prior to conducting the present clinical study, a methodological study absent of drug treatment had been performed to evaluate experimental techniques and identify possible sources of analytical, biological, and experimental variability that might occur during the assessment of analytes in the CSF (Patil et al. 2007a). Analytical methods were established with quantitative dynamic ranges and limits of quantification for the anticipated endogenous concentrations of each analyte. Cerebrospinal fluid was obtained by lumbar puncture at baseline and 2 weeks after baseline in healthy subjects. Aliquots of CSF were analyzed for the same biogenic amines and metabolites evaluated in the current study. The ratios of CSF monoamine and metabolite concentrations determined 2 weeks after baseline compared with baseline ranged from 1.01 to 1.02 for DOPAC, HVA, DHPG, and MHPG and from 1.11 to 1.17 for L-DOPA, 5-HTP, and 5-HIAA. Therefore, the temporal biological stability of monoamines and monoamine metabolites in human CSF was considered sufficient to support the investigation of drug or placebo treatment effect. Overall, the results of the clinical study provide the first demonstration of an effect of an agonist at metabotropic glutamate 2,3 receptors on biogenic amine systems in humans. The exact mechanism whereby LY2140023 monohydrate increases dopaminergic and serotonergic turnover is unknown. However, the results from the clinical study are consistent with preclinical studies with this and other mGlu2/3 agonists, including published microdialysis and brain tissue data for LY404039 (Rorick-Kehn et al. 2007) and the mGlu2/3 agonist LY379268 (Cartmell et al. 2000). Therefore, in both humans and in the preclinical setting, the doses of LY2140023 monohydrate (and/or LY404039) that were found to induce biogenic amine metabolite changes in the CSF (and/or microdialysate) were comparable to doses that were associated with evidence of efficacy in either schizophrenia or an animal model of schizophrenia. The similarity of result from the studies in rats and humans using varied analytical techniques (GC/MS/MS versus HPLC/EC) and distinctly different biological matrices (dialysates from brain parenchymal interstitial fluid in rats, cistern magna CSF in rats, and lumbar CSF in humans) further supports the notion that the observed changes in human lumbar CSF neurotransmitter metabolite concentrations are indeed indicative of a central clinical pharmacodynamic effect. The LY2140023 monohydrate treatment-associated increases in biogenic amines in rodents (DOPAC and HVA) and healthy subjects (DOPAC, HVA, and 5-HIAA) suggest that LY2140023 may particularly increase dopamine and possibly serotonin turnover in the PFC, a finding observed in preclinical studies with atypical antipsychotics including olanzapine (Li et al. 1998). Unlike atypical antipsychotics, however, LY2140023 and the active agent LY404039 do not directly interact with the dopamine D2 receptor (Fell et al. 2008). The observed effects on biogenic amine metabolite turnover are therefore presumed to be mediated indirectly via changes in glutamate release. While there was an increase in CSF MHPG with LY404039, no clear conclusions can be made regarding the effect of LY2140023 on NE turnover as no statistical significant change in CSF DHPG concentrations was observed. CSF and plasma Pomaglumetad methionil/LY2140023 and LY404039 concentrations following 14 days of LY2140023 monohydrate BID administration in the present study were within the same range of concentrations previously reported in studies of healthy subjects administered 40-mg doses (data not shown). Furthermore, individual concentrations of the active compound LY404039 in both CSF and plasma increased linearly with increasing CSF concentrations of HVA. A similar trend for increasing concentrations of HVA and DOPAC was seen in the rat, both for CSF and plasma concentrations of LY404039. Taken together, the association of biogenic amine concentrations with drug levels further supports the notion that the observed pharmacodynamic changes are in response to metabotropic glutamate 2,3 activation by LY404039, the active component of LY2140023 monohydrate. Furthermore, the relationship between central pharmacodynamic markers and peripheral LY404039 levels suggest that plasma LY404039 concentrations may predict central pharmacokinetic and pharmacodynamic effects. If confirmed in future studies, plasma LY404039 determinations may suffice to reflect central pharmacokinetic and central pharmacodynamic activity, with the latter based on biogenic amine neurotransmitter turnover. Further studies are required to evaluate this possibility and define the relationship to clinical outcome, if such a relationship exists. In conclusion, Pomaglumetad methionil/LY2140023 and/or LY404039 increased CNS DA turnover and possibly also increased 5-HT turnover, as reflected by the increased CSF concentrations of the metabolites derived from these neurotransmitters. The effects of LY2140023 and/or LY404039 on these markers are presumed to be mGlu2/3 receptor-mediated, so the measurement of DA and 5-HT metabolites may serve as useful translational markers of LY2140023 and/or LY404039 central pharmacodynamic activity. The lack of any significant affinity of LY2140023 or LY404039 for dopamine and serotonin receptors suggests that the impact on DA and 5-HT turnover is likely mediated through an indirect mechanism. [1] Despite peptide transporter 1 (PEPT1) being responsible for the bioavailability for a variety of drugs, there has been little study of its potential involvement in drug-drug interactions. Pomaglumetad methionil, a metabotropic glutamate 2/3 receptor agonist prodrug, utilizes PEPT1 to enhance absorption and bioavailability. In vitro studies were conducted to guide the decision to conduct a clinical drug interaction study and to inform the clinical study design. In vitro investigations determined the prodrug (Pomaglumetad methionil/LY2140023 monohydrate) is a substrate of PEPT1 with Km value of approximately 30 µM, whereas the active moiety (LY404039) is not a PEPT1 substrate. In addition, among the eight known PEPT1 substrates evaluated in vitro, valacyclovir was the most potent inhibitor (IC50 = 0.46 mM) of PEPT1-mediated uptake of the prodrug. Therefore, a clinical drug interaction study was conducted to evaluate the potential interaction between the prodrug and valacyclovir in healthy subjects. No effect of coadministration was observed on the pharmacokinetics of the prodrug, valacyclovir, or either of their active moieties. Although in vitro studies showed potential for the prodrug and valacyclovir interaction via PEPT1, an in vivo study showed no interaction between these two drugs. PEPT1 does not appear to easily saturate because of its high capacity and expression in the intestine. Thus, a clinical interaction at PEPT1 is unlikely even with a compound with high affinity for the transporter. [2] Subsequently, a clinical study was designed to evaluate Pomaglumetad methionil/LY2140023 as both a substrate and an inhibitor of PEPT1. The coadministration of LY2140023 and valacyclovir did not affect the PK of each other or their respective active moieties (LY404039 or acyclovir), indicating no clinical DDI between the prodrug and valacyclovir. The data also showed that the presence of the prodrug or valacyclovir did not affect the conversion of prodrug to its active moiety for either LY2140023 or for valacyclovir. The lack of interaction on the conversion of the prodrug and valacyclovir to its corresponding active moieties was expected, because different enzymes are responsible for their activation. Dehydropeptidase 1 has been shown to cleave the prodrug to its active moiety (Moulton et al., 2015) and valacyclovirase (biphenyl hydrolase-like protein) to cleave valacyclovir to acyclovir (Marsillach et al., 2014). For both active moieties there was no change in the CL/F or the CLr, indicating that coadministration of the drugs did not affect the renal clearance of each other. Furthermore, if there is a weak interaction at PEPT1, a shift in Tmax values may be observed. However, no shift in Tmax was observed for any of the entities studied. Also, for valacyclovir there was no significant change in the AUC(0–3 hours), again indicating no interaction at PEPT1. In this study, we illustrated how in vitro studies can guide the design of clinical DDI studies for transporter-based interactions. In vitro screening of inhibitory potencies of multiple drugs that compete at the transporter could give the rank order of inhibitory potencies and an analysis for potential for DDI in relation to oral dose of the compound. Therefore, unnecessary in vivo studies could be avoided, while focusing on the most relevant potential for DDI. Although the in vitro study indicated the potential for a DDI between the prodrug and valacyclovir according to guideline for other intestinal transporters, an in vivo DDI study showed no interaction of these two drugs via PEPT1. Therefore, our results clearly illustrated that a clinical DDI at PEPT1 is highly unlikely even with a NME with high affinity for the transporter.[2] |
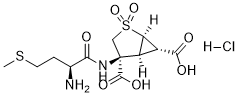
| 分子式 |
C12H19CLN2O7S2
|
|---|---|
| 分子量 |
402.861
|
| 精确质量 |
402.032
|
| 元素分析 |
C, 35.78; H, 4.75; Cl, 8.80; N, 6.95; O, 27.80; S, 15.92
|
| CAS号 |
635318-26-2
|
| 相关CAS号 |
Pomaglumetad methionil;956385-05-0;Pomaglumetad methionil anhydrous;635318-55-7
|
| PubChem CID |
66903228
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| tPSA |
198
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
644
|
| 定义原子立体中心数目 |
5
|
| SMILES |
CSCC[C@@H](C(=O)N[C@]1(CS(=O)(=O)[C@@H]2[C@H]1[C@H]2C(=O)O)C(=O)O)N.Cl
|
| InChi Key |
KTYRTJLEXFSWRJ-LBMFEJOUSA-N
|
| InChi Code |
InChI=1S/C12H18N2O7S2.ClH/c1-22-3-2-5(13)9(15)14-12(11(18)19)4-23(20,21)8-6(7(8)12)10(16)17;/h5-8H,2-4,13H2,1H3,(H,14,15)(H,16,17)(H,18,19);1H/t5-,6+,7+,8-,12-;/m0./s1
|
| 化学名 |
(1R,4S,5S,6S)-4-[[(2S)-2-amino-4-methylsulfanylbutanoyl]amino]-2,2-dioxo-2λ6-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic acid;hydrochloride
|
| 别名 |
Prodrug of LY-404039; Pomaglumetad methionil HCl; LY-2140,023; LY2140,023 hydrochloride; 635318-26-2; Pomaglumetad methionil (hydrochloride); Pomaglumetad methionil hydrochloride; Pomaglumetad methionil HCl; SCHEMBL1089235; LY2140023 (hydrochloride); LY 2140023; LY2140023; LY2140023 monohydrate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~25 mg/mL (~62.05 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.21 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.21 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.21 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4823 mL | 12.4113 mL | 24.8225 mL | |
| 5 mM | 0.4965 mL | 2.4823 mL | 4.9645 mL | |
| 10 mM | 0.2482 mL | 1.2411 mL | 2.4823 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study
Estudio de Fase 2, de 17 Semanas, Multicéntrico, Aleatorizado y Doble Ciego, Sobre la Eficacia de LY2140023 Combinado con Tratamiento Clínico Habitual Comparado con Placebo Combinado con Tratamiento Clínico Habitual, en Pacientes con Esquizofrenia con Síntomas Negativos Prominentes
CTID: null
Phase: Phase 2 Status: Completed
Date: 2010-02-02