| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed; Bioavailability is approximately 96%. Primaquine is well absorbed from the GI tract. Following oral administration, peak plasma concentrations of the drug generally are attained within 6 hours; plasma concentrations generally are negligible after 24 hours. Considerable interindividual variation in peak plasma concentrations of primaquine have been reported with the same dose of the drug. Although specific information on the distribution of primaquine into body tissues and fluids is not available, the drug appears to be widely distributed in the body following oral administration. Primaquine has an apparent volume of distribution of about 150 to 250 L in healthy adults. Extensively distributed; ... the whole-blood-to-plasma distribution ratio was 0.93 in one study of patients being treated with 15 mg (base) daily for 14 days. For more Absorption, Distribution and Excretion (Complete) data for PRIMAQUINE (7 total), please visit the HSDB record page. Metabolism / Metabolites The principal metabolite of primaquine is carboxyprimaquine, and plasma concentrations of the metabolite greatly exceed those of unchanged primaquine. It is not known whether /carboxyprimaquine/ has antimalarial activity. Primaquine is rapidly metabolized ... Three identified oxidative metabolites ... are 8-(3-carboxyl-1-methylpropylamino)-6-methoxyquinoline, 5-hydroxy primaquine, and 5-hydroxy-6-desmethylprimaquine. The carboxyl derivative is the major metabolite found in human plasma. After a single dose it reaches concentrations in plasma more than 10 times those of primaquine; this nontoxic metabolite also is eliminated more slowly and accumulates with multiple doses ... The 3 metabolites ... appear to have appreciably less antimalarial activity than does primaquine. However, except for the carboxyl derivative, their hemolytic activity, as assessed by formation of methemoglobin in vitro, is greater than that of the parent compound. Biological Half-Life 3.7-7.4 hours Primaquine has a plasma half-life of 3.7-9.6 hours in healthy adults. /The half-life of/ carboxyprimaquine is 22 to 30 hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Despite use for more than 50 years, primaquine has not been linked to significant serum aminotransferase elevations or to clinically apparent acute liver injury. Primaquine can cause hemolysis in patients with G6PD deficiency, which can result in mild jaundice. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Primaquine and its metabolite are poorly excreted into breastmilk of nursing mothers and undetectable in the serum of their breastfed infants. Breastfed infants beyond the neonatal period have shown no evidence of hemolysis. Neonates and infants with glucose-6-phosphate dehydrogenase (G6PD) deficiency have not been studied, but G6PD-deficient infants over 28 days of age appear to have a low risk of hemolysis from exposure in breastmilk. If primaquine is required, testing the mother and infant for G6PD deficiency is advisable before the drug is given to a nursing mother. United Kingdom malaria treatment guidelines recommend that primaquine be avoided in nursing mothers with malaria and that weekly chloroquine 500 mg be given until breastfeeding is completed. However, these guidelines were developed before information on the excretion of primaquine into breastmilk and safety in breastfed infants was published. More recent information indicates that all mothers nursing infant over 28 days of age could safely receive primaquine. The Centers for Disease Control and Prevention guidelines state that primaquine may be used in breastfeeding mothers and infants with normal G6PD levels. Because the small amounts of primaquine transferred in breast milk are insufficient to provide adequate protection or treatment of malaria, infants who require chemoprophylaxis or therapy must receive the recommended dosages of primaquine. ◉ Effects in Breastfed Infants Twenty-one mothers with vivax malaria were give a dosage of primaquine 0.5 mg/kg daily for 14 days while breastfeeding their infants who were at least 28 days old. No alterations in hematocrit, Heinz body counts, serum bilirubin, oxygen saturation, or methemoglobinemia were seen in any of the infants. A woman with vivax malaria who was 5 months postpartum was given a dose of primaquine of 0.52 mg/kg daily for 7 days, then 0.46 mg/kg daily for 7 days after rechecking the patient’s weight. Shwas found to be heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency and experienced some hemolysis and anemia. Her female infant was being breastfed (extent not stated) during treatment and was found to be heterozygous for the G6PD Mahidol variant, but had no apparent hemolysis. The child’s vaccination schedule was completed, and the 6-month motor milestones were normal. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 其他信息 |
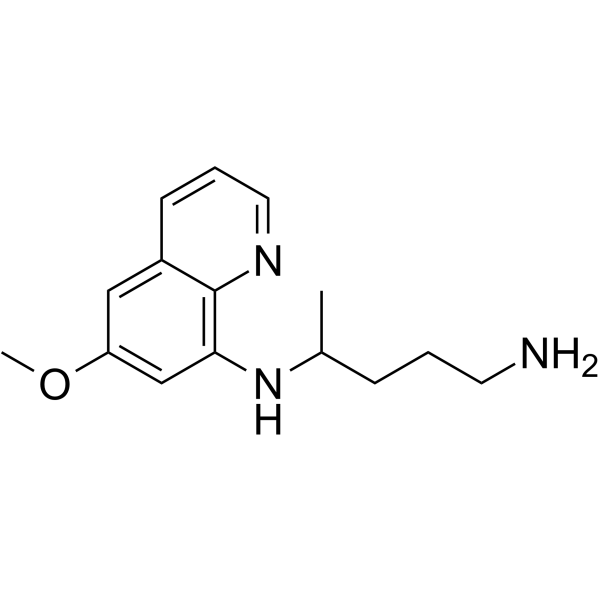
Primaquine is an N-substituted diamine that is pentane-1,4-diamine substituted by a 6-methoxyquinolin-8-yl group at the N(4) position. It is a drug used in the treatment of malaria and Pneumocystis pneumonia. It has a role as an antimalarial. It is an aminoquinoline, a N-substituted diamine and an aromatic ether.
Primaquine phosphate is an antimalarial prescription medicine approved by the U.S. Food and Drug Administration (FDA) for preventing relapses of malaria caused by the parasite Plasmodium vivax (P. vivax). Malaria can be an opportunistic infection (OI) of HIV. An OI is an infection that occurs more frequently or is more severe in people with weakened immune systems—such as people with HIV—than in people with healthy immune systems. An aminoquinoline that is given by mouth to produce a radical cure and prevent relapse of vivax and ovale malarias following treatment with a blood schizontocide. It has also been used to prevent transmission of falciparum malaria by those returning to areas where there is a potential for re-introduction of malaria. Adverse effects include anemias and GI disturbances. (From Martindale, The Extra Pharmacopeia, 30th ed, p404) Primaquine is an Antimalarial. Primaquine is an aminoquinoline that has been used for the prevention and therapy of malaria for more than 50 years. Primaquine is not associated with serum enzyme elevations during therapy and has yet to be linked to instances of clinically apparent acute liver injury. Primaquine has been reported in Streptomyces rimosus with data available. Primaquine is a synthetic, 8-aminoquinoline derivative with antimalarial properties. Although its mechanism of action is unclear, primaquine bind to and alter the properties of protozoal DNA. This agent eliminates tissue (exo-erythrocytic) malarial infection, preventing the development of the erythrocytic forms of the parasite which are responsible for relapses in Plasmodium vivax and ovale malaria. Primaquine is active against late hepatic stages (hypnozoites, schizonts). (NCI04) An aminoquinoline that is given by mouth to produce a radical cure and prevent relapse of vivax and ovale malarias following treatment with a blood schizontocide. It has also been used to prevent transmission of falciparum malaria by those returning to areas where there is a potential for re-introduction of malaria. Adverse effects include anemias and GI disturbances. (From Martindale, The Extra Pharmacopeia, 30th ed, p404) See also: Primaquine Phosphate (has salt form). Drug Indication For the treatment of malaria. FDA Label Mechanism of Action Primaquine's mechanism of action is not well understood. It may be acting by generating reactive oxygen species or by interfering with the electron transport in the parasite. Also, although its mechanism of action is unclear, primaquine may bind to and alter the properties of protozoal DNA. The precise mechanism of action has not been determined, but may be based on primaquine's ability to bind to and alter the properties of DNA. Primaquine is highly active against the exoeryhrocytic stages of plasmodium vivax and plasmodium ovale and against the primary exoerythrocytic stages of plasmodium falciparum. It is also highly active against the sexual forms of (gametocytes) plasmodia, especially P. falciparum, disrupting transmission of the disease by eliminating the reservoir from which the mosquito carrier is infected. /Primaquine/ disrupts the parasitic mitochondria, thereby interrupting metabolic processes requiring energy. ... /Primaquine is one/ of /aromatic amine-containing/ xenobiotics ... capable to inducing oxidative injury in erythrocytes. These agents appear to potentiate the normal redox reactions and are capable of overwhelming the usual protective mechanisms. The interaction between these xenobiotics and hemoglobin leads to the formation of free radicals that denature critical proteins, including hemoglobin, thiol-dependent enzymes, and components of the erythrocyte membrane ... Oxidative denaturation of the globin chain decreases its affinity for the heme group, which may dissociate from the globin chain during oxidative injury ... The generation of free radicals may also lead to peroxidation of membrane lipids. This may affect the deformability of the erythrocyte and the permeability of the membrane to potassium. The alteration of the Na(+)/K(+) gradient is ... potentially lethal to the affected erythrocyte. Oxidative injury also impairs the metabolic machinery of the erythrocyte, resulting in a decrease in the concentration of ATP. Damage to the membrane can also permit leakage of denatured hemoglobin from the cell. Such free denatured hemoglobin can be toxic on its own. Free hemoglobin may irreversibly bind nitric oxide, resulting in vasoconstriction. Released hemoglobin may form nephrotoxic hemoglobin dimers, leading to kidney damage. /Oxidative hemolysis/ |
| 分子式 |
C15H21N3O
|
|---|---|
| 分子量 |
259.34674
|
| 精确质量 |
259.168
|
| CAS号 |
90-34-6
|
| 相关CAS号 |
Primaquine diphosphate;63-45-6;Primaquine-13C,d3
|
| PubChem CID |
4908
|
| 外观&性状 |
Viscous liquid
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
451.1±45.0 °C at 760 mmHg
|
| 熔点 |
25°C
|
| 闪点 |
226.6±28.7 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.616
|
| LogP |
2.67
|
| tPSA |
60.17
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
262
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1C2C(=CC(=CC=2NC(CCCN)C)OC)C=CC=1
|
| InChi Key |
INDBQLZJXZLFIT-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H21N3O/c1-11(5-3-7-16)18-14-10-13(19-2)9-12-6-4-8-17-15(12)14/h4,6,8-11,18H,3,5,7,16H2,1-2H3
|
| 化学名 |
4-N-(6-methoxyquinolin-8-yl)pentane-1,4-diamine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~963.95 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (8.02 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8558 mL | 19.2790 mL | 38.5579 mL | |
| 5 mM | 0.7712 mL | 3.8558 mL | 7.7116 mL | |
| 10 mM | 0.3856 mL | 1.9279 mL | 3.8558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。