| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
BTK (IC50 = 1.3 nM); BMX (IC50 = 1.0 nM); ITK (IC50 = 440 nM); TEC (IC50 = 0.8 nM); RLK (IC50 = 1.2 nM); BLK (IC50 = 6.3 nM); EGFR (IC50 = 520 nM); ERBB2 (IC50 = 3900 nM); ERBB4 (IC50 = 11.3 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
rilzabrutinib 是布鲁顿酪氨酸激酶 (BTK) 的可逆共价抑制剂,IC50 为 1.3±0.5 nM。此外,当针对 251 种其他激酶进行评估时,发现 rilzabrutinib 具有极高的选择性。 Rilzabrutinib以BTK的半胱氨酸为靶点,导致解离速率延迟;化学物质在体外被洗掉 18 小时后,79±2% 的结合 BTK 仍然存在于 PBMC 中。共价半胱氨酸结合的完全可逆性发生在靶标变性时。 Rilzabrutinib 抑制抗 IgM 和 B 细胞 CD69 表达产生的人 B 细胞增殖(10% 血清),IC50 值分别为 5±2.4 nM 和 123±38 nM [2]。
|
||
| 体内研究 (In Vivo) |
药物从血流中去除后,利扎布替尼继续具有与延长的目标停留时间一致的药效作用。此外,rilzabrutinib 以剂量依赖的方式逆转并完全抑制大鼠胶原诱导的关节炎,这将靶点占用与疾病的缓解相关联 [2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Cmax was highly correlated with the magnitude of BTK occupancy 4 h post‐PRN1008 dosing (Figure 4). A sigmoidal Emax model, with a fitted γ and E0 provided the best fit of PRN1008 Cmax vs. occupancy data. The parameter estimates (% coefficient of variation) were: Emax 76.7 (13) %; EC50 46.5 (15) ng ml–1; E0 17.8 (42) %, and γ 2.1 (31). These results suggest a fairly steep exposure–response relationship, with low variability, and with 80% of maximal occupancy achieved at Cmax concentrations of approximately 100 ng ml–1 and above. The E0 value of 17.8% is consistent with the lower quantifiable range of the assay.
The robust relationship between PRN1008 Cmax and 4‐h occupancy, along with the very consistent occupancy decay rate of ~1.6% h–1 should allow for design of dosing regimens (dose and dose intervals) which precisely target various levels of occupancy over the course of a dose interval. It is noted that the nature of the PK and PK/PD relationships of PRN1008 may differ between healthy volunteers and patient populations, and should be further evaluated in future studies in patients.[1] This single-center, open-label, non-randomized, two-part, phase I study was conducted (1) to evaluate the absolute oral bioavailability of rilzabrutinib 400 mg tablet following an i.v. microtracer dose of ~100 μg [14C]-rilzabrutinib (~1 μCi) and single oral dose of 400 mg rilzabrutinib tablet (part 1), and (2) to characterize the absorption, metabolism, and excretion (AME) of 14C-radiolabeled rilzabrutinib following single oral dose (300 mg) of [14C]-rilzabrutinib (~1000 μCi; administered as a liquid) in healthy male participants (part 2). A total of 18 subjects were enrolled (n = 8 in part 1; n = 10 in part 2). The absolute bioavailability of 400 mg rilzabrutinib oral tablet was low (<5%). In part 1, rilzabrutinib was absorbed rapidly after single oral dose of rilzabrutinib 400 mg tablet with a median (range) time to maximum concentration (Tmax ) value of 2.03 h (1.83-2.50 h). The geometric mean (coefficient of variation) terminal half-life following the oral dose and i.v. microtracer dose of ~100 μg [14C]-rilzabrutinib, were 3.20 (51.0%) and 1.78 (37.6%) h, respectively. In part 2, rilzabrutinib was also absorbed rapidly following single oral dose of 300 mg [14C]-rilzabrutinib solution with a median (range) Tmax value of 1.00 h (1.00-2.00 h). The majority of total radioactivity was in the feces for both non-bile collection subjects (92.9%) and bile collection subjects (87.6%), and ~5% of radioactivity was recovered in urine after oral administration. Urinary excretion of unchanged rilzabrutinib was low (3.02%). The results of this study advance the understanding of the absolute bioavailability and AME of rilzabrutinib and can help inform its further investigation.Clin Transl Sci. 2023 Jul;16(7):1210-1219. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Data from all 80 enrolled subjects who received study drug (either PRN1008 or placebo) were included in the safety population. All participants were assessed for AEs for the duration of the study. No serious AEs or deaths were reported during the study, and no participants discontinued treatment due to an AE in either Part A or Part B.
At PRN1008 single doses of 50 to 600 mg, safety and tolerability was similar to placebo. In these four cohorts, there was only one subject of the six treated in each cohort who experienced a treatment‐emergent AE (TEAE). Of these four TEAEs, only one was considered related to study drug (nausea in Cohort A4), and one was graded as moderate (toothache in Cohort A2, unrelated to study drug). This compares with two TEAEs reported in two of the 10 subjects who received placebo (both graded as mild, not drug related).
In Cohort A5 (1200 mg), the primary AEs observed were gastrointestinal (GI) in nature, and were reported by each of the six subjects receiving PRN1008. The drug‐related AEs included diarrhoea (coded as loose stools; n = 6, 3 mild and 3 moderate severity), nausea (n = 3, 2 mild and 1 moderate severity), vomiting (n = 1), throat irritation (n = 3), and oropharyngeal discomfort (n = 1).
There was no apparent relationship between GI AEs and PRN1008 pharmacokinetics. As described above, both Cmax and area under the concentration–time curve for PRN1008 were similar at the 600 mg and 1200 mg doses. Despite similar plasma PK, the increase in GI AEs for the 600 mg vs. 1200 mg dose levels would suggest a localized effect related to total administered dose, and not to plasma exposure.
Following 10 or 11 days of dosing, PRN1008 was generally safe and well tolerated. TEAEs were reported in 7/8, 4/8, 8/8, 7/8 and 4/8 subjects in the 300 mg QD, 300 mg twice daily (BID), 600 mg QD, 450 mg BID and placebo groups, respectively. TEAEs classified as treatment‐related appeared to be more frequent in PRN1008 receiving subjects, reported in 6/8, 3/8, 8/8, 6/8 and 1/8 subjects in the 300 mg QD, 300 mg BID, 600 mg QD, 450 mg BID and placebo groups, respectively. All TEAEs classified as related to study drug were mild in intensity, with the exception of one subject in the 450 mg BID cohort who reported moderate diarrhoea.
There were no clinically significant or dose‐dependent changes observed in haematology, biochemistry or coagulation laboratory parameters in either Part A or Part B of the study. Similarly, no clinically significant changes were observed in vital signs, ECGs or urinalysis evaluations.[1]
|
||
| 参考文献 |
|
||
| 其他信息 |
Rilzabrutinib is an oral, reversible covalent inhibitor of Bruton's tyrosine kinase being investigated for the treatment of immune disorders, such as immune thrombocytopenic purpura.
Rilzabrutinib is an orally bioavailable reversible covalent inhibitor of Bruton's tyrosine kinase (BTK), with potential immunomodulatory and anti-inflammatory activities. Upon oral administration, rilzabrutinib inhibits the activity of BTK. This prevents the activation of the B-cell antigen receptor (BCR) signaling pathway, and the resulting immune activation and inflammation. BTK, a cytoplasmic tyrosine kinase and member of the Tec family of kinases, plays an important role in B-lymphocyte development, activation, signaling, proliferation and survival. In addition to B-cells, BTK is also expressed in other cells of hematopoietic origin, including monocytes, macrophages, neutrophils, mast cells, eosinophils and platelets, and plays an important role in both adaptive and innate immune responses. Drug Indication Treatment of immune thrombocytopenia |
| 分子式 |
C36H40FN9O3
|
|
|---|---|---|
| 分子量 |
665.7597
|
|
| 精确质量 |
665.32
|
|
| 元素分析 |
C, 64.95; H, 6.06; F, 2.85; N, 18.93; O, 7.21
|
|
| CAS号 |
1575596-29-0
|
|
| 相关CAS号 |
1575596-77-8;1575596-29-0;1575591-66-0
|
|
| PubChem CID |
73388818
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
3.4
|
|
| tPSA |
139
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
49
|
|
| 分子复杂度/Complexity |
1230
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
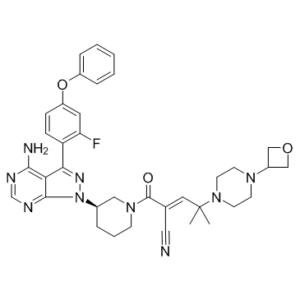
CC(C)(/C=C(\C#N)/C(=O)N1CCC[C@H](C1)N2C3=NC=NC(=C3C(=N2)C4=C(C=C(C=C4)OC5=CC=CC=C5)F)N)N6CCN(CC6)C7COC7
|
|
| InChi Key |
LCFFREMLXLZNHE-GBOLQPHISA-N
|
|
| InChi Code |
InChI=1S/C36H40FN9O3/c1-36(2,45-15-13-43(14-16-45)26-21-48-22-26)18-24(19-38)35(47)44-12-6-7-25(20-44)46-34-31(33(39)40-23-41-34)32(42-46)29-11-10-28(17-30(29)37)49-27-8-4-3-5-9-27/h3-5,8-11,17-18,23,25-26H,6-7,12-16,20-22H2,1-2H3,(H2,39,40,41)/b24-18+/t25-/m1/s1
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.12 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.12 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.12 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5020 mL | 7.5102 mL | 15.0204 mL | |
| 5 mM | 0.3004 mL | 1.5020 mL | 3.0041 mL | |
| 10 mM | 0.1502 mL | 0.7510 mL | 1.5020 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Individual BTK occupancy by PRN1008 dose level (Part A). Solid line represents fit of a linear regression model to estimate loss of occupancy over time.Br J Clin Pharmacol.2017 Nov;83(11):2367-2376. |
|---|
Duration of BTK occupancy (squares) in relation to the plasma concentration profile of PRN1008 (circles), following final dose on day 10 of a 600mg once daily dosing regimen in the multiple ascending dose study.Br J Clin Pharmacol.2017 Nov;83(11):2367-2376. |
Exposure–response relationship between 4‐hour BTK occupancy and PRN1008 maximum observed concentration (Part A).Br J Clin Pharmacol.2017 Nov;83(11):2367-2376. |