| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Alpha 2-adrenoceptor
|
|---|---|
| 体外研究 (In Vitro) |
2-Methoxyidazoxan(RX 8210022)是一种高度选择性的α2-肾上腺素受体拮抗剂,几乎没有咪唑啉拮抗剂作用。RX821002是一种通过阻断蓝斑中的α2肾上腺素受体在皮质中引发去甲肾上腺素释放的药物[1]。
|
| 体内研究 (In Vivo) |
2-Methoxyidazoxan HCl/RX 821002(1 mg/kg) 可提高患有新生儿腹侧海马病变 (NVHL) 的大鼠在新环境中活动的能力。 2-2-甲基咪唑克单盐酸盐对运动的影响是双相的,首先表现出减弱,然后增强[3]。
患有新生儿腹侧海马损伤(NVHL)的大鼠用于建立精神分裂症模型。它们在青春期后表现出更强的运动能力和学习困难。多巴胺能精神兴奋剂药物加强了这种行为改变,这也与精神分裂症有关,因为它说明了其多巴胺能方面。但只有多巴胺能药物才能产生这种作用,这仍然值得怀疑。行为效应可能只是代表一种非特异性的唤醒,在这种情况下,NVHL大鼠也应该对其他提高警惕的药物有高度反应。我们分别以5mg/kg和1mg/kg的剂量给药腺苷(咖啡因)或肾上腺素受体拮抗剂(RX 821002),以改变大鼠的警觉性。在实验前使用磁共振成像(MRI)选择大鼠。每组都有典型和相似的NVHL病变。将它们与假损伤大鼠进行比较。我们评估了在新环境中的运动能力,以及记忆宣布食物出现的视觉或听觉线索的能力。咖啡因和RX82100都能增强新环境中的运动能力,特别是在NVHL大鼠中。但是,RX82100对运动有双相影响,包括在增强之前的初始减少。它与病变无关。咖啡因不会改变NVHL大鼠的学习表现。但是,RX 821002被发现有助于学习。患者往往比健康人摄入更多的咖啡因,这被解释为一种对抗一些认知缺陷的方法。这一想法没有得到目前结果的验证。但肾上腺素能药物可能有助于减轻他们的一些认知缺陷[3]。 |
| 酶活实验 |
检查了四种拮抗剂区分α2A和直系α2D肾上腺素受体的能力。拮抗剂为(2S,12bS)1',3'-二甲基螺环(1,3,4,5',6,6',7,12b-八氢-2H-苯并[b]呋喃并[2,3-a]喹啉嗪)-2,4'-嘧啶-2'-酮(MK912)、2-[2-(甲氧基-1,4-苯并二恶烷基)咪唑啉(RX 821002)依法氧嘧啶和苯氧噻嗪。兔大脑皮层中的α2受体被选为α2A,豚鼠大脑皮层中为α2D肾上腺素受体。大脑皮层切片用3H-去甲肾上腺素预孵育,然后用短暂的脉冲串(4个脉冲,100 Hz)进行超灌注和电刺激,即使有α2-自抑制作用,也很少。5-溴-6-(2-咪唑啉-2-基氨基)喹喔啉(UK 14304)用作α2-肾上腺素受体激动剂。UK 14304降低了刺激诱发的氚溢出。拮抗剂以明显竞争的方式将UK 14304的浓度抑制曲线向右移动。根据位移计算拮抗剂的解离常数。MK 912,RX 821002 依法沙星对(豚鼠)α2D肾上腺素受体的亲和力(pKd值分别为10.0、9.7和9.1)明显高于(兔)α2A肾上腺素受体(pKd分别为8.9、8.2和7.6)。苯氧噻嗪对α-2A-(pKd 7.4)的亲和力高于对α-2D肾上腺素受体(pKd 6.9)的亲和力。根据α2A和α2D之间的四种化合物的Kd值计算的比率高达100倍。结论是MK 912,RX 821002,依法氧嘧啶和苯氧噻嗪是具有高能力区分α2A和α2D肾上腺素受体的拮抗剂[2]。
|
| 动物实验 |
Selecting subjects using MRI imaging techniques[3]
Twenty-one day-old lesioned pups were subjected to an MRI session under isoflurane anesthesia. MRI was performed on a small-animal scanner operating at 4.7 T (TR/TE/TEeff: 3000/30 ms/60 ms). A series of 10 slices (256 × 256 pixels) was generated over a 1 cm long section of the brain, rostral to the cerebellum-cerebrum gap, as in our previous studies and those conducted by others (Angst et al., 2007; Macedo et al., 2008, 2010, 2012; Bertrand et al., 2010; Sandner et al., 2010, 2011, 2012), the purpose being to select triplets of lesioned rats (1 saline, 1 caffeine and 1 RX 821002), where each member of the triplet had about the same MRI image in terms of the location and symmetry of the lesion (examples are shown in Figure 1). We obtained 9 triplets of lesions (27 lesioned rats), to which we added 27 sham-lesioned controls. Rats that could not be included in a triplet were transferred to other research protocols. Lesioned areas were drawn on MRI coronal sections. The numbers of pixels of the left and right lesions were summed up over successive rostro-caudal sections. The sum represents then the estimated volumes of the lesions. It was submitted to an ANOVA, with lesion side as within-group factor and treatment as between-group factor. Another ANOVA was computed on the sum of left and right lesions with the three rats in each triplet as within-group factor. The threshold for statistical significance for all statistical computations was set to p < 0.05. Treatments[3] A 3 × 2 experimental design was used (6 groups of 9 rats). The treatment was applied before each test and each learning session. The latency between injection and the beginning of the test was 10 min for caffeine (5 mg/kg) and 20 min for RX 821002 (1 mg/kg), dissolved in saline (vehicle: veh) in a final volume of 1 ml and injected i.p. Control rats received a saline injection 10 or 20 min before testing. The following groups were considered: 9 NVHL rats treated with caffeine (caf group), 9 NVHL rats treated with RX 821002 (RX group), and 9 NVHL rats which were given saline (veh group), plus three groups of 9 sham-lesioned rats which received the same treatments. |
| 参考文献 |
|
| 其他信息 |
Adrenergic alpha-Antagonists:
Drugs that bind to but do not activate alpha-adrenergic receptors thereby blocking the actions of endogenous or exogenous adrenergic agonists. Adrenergic alpha-antagonists are used in the treatment of hypertension, vasospasm, peripheral vascular disease, shock, and pheochromocytoma.
Increasing evidence indicates that the hypotensive effect of centrally acting antihypertensive drugs is not due to stimulation of alpha 2-adrenoceptors but to action on imidazoline receptors (IR). This has led to the development and recent clinical use of second generation agents such as rilmenidine and moxonidine that possess a much greater selectivity toward these nonadrenergic receptors. However, relatively few studies have examined the role of these receptors in conscious animals or have adequately accounted for the alpha 2-adrenoceptor antagonist properties of IR antagonists such as idazoxan. We have taken the approach of initially calibrating the alpha 2-adrenoceptor antagonist potency of intracisternally (ic) administered idazoxan and the IR-1 receptor antagonist efaroxan against 2-methoxyidazoxan, a highly selective alpha 2-adrenoceptor antagonist with little or no imidazoline antagonist effect. This was done using alpha-methyldopa, a hypotensive agent affecting only alpha 2-adrenoceptors. Thus, we chose doses of the antagonists with equal alpha 2-adrenoceptor blocking action such that differences in the ability of idazoxan or efaroxan compared to 2-methoxy-idazoxan to reverse the hypotension produced by rilmenidine, moxonidine, or clonidine indicate an interaction with IR. By this method we found that the hypotensive effects of rilmenidine and moxonidine at moderate intracisternal doses were more readily reversed by the imidazoline antagonists than by 2-methoxy-idazoxan, indicating that IR were largely responsible for their hypotensive actions. By contrast, clonidine's effects were equally reversed by all antagonists, suggesting interaction mainly with alpha 2-adrenoceptors. In conscious rabbits with chronic renal sympathetic nerve electrodes we examined the effect of rilmenidine and alpha-methyldopa on the renal sympathetic baroreflex. Both drugs reduced renal sympathetic nerve activity and sympathetic baroreflex responses, but only the effect of rilmenidine was preferentially reversed by idazoxan. Thus, both IR and central alpha 2-adrenoceptor receptors can influence the renal baroreflex, but the former are relatively more important for the actions of rilmenidine. We recently examined the possible sites of action of rilmenidine in anesthetized rabbits and showed that sixfold lower doses were required to reduce blood pressure when the drug was injected into the rostral ventrolateral medulla compared to intracisternal administration. At this site rilmenidine also reduced renal sympathetic tone and inhibited renal sympathetic baroreflex responses. By contrast, rilmenidine was relatively ineffective when injected into the nucleus of the solitary tract. These experiments support the view that rilmenidine acts primarily at IR in the rostral ventrolateral medulla to reduce sympathetic tone and modulate sympathetic baroreflexes.[1] Contrasting with the lack of improvement in the performance of rats under caffeine, RX821002, the alpha2-adrenoreceptor antagonist, improved learning. Research about the contribution of noradrenergic systems to schizophrenia has yielded inconsistent results (van Kammen and Antelman, 1984; van Kammen and Kelley, 1991; Yamamoto et al., 1994; Friedman et al., 1999; Klimek et al., 1999). Interest has been shown, however, in the prefrontal noradrenergic mechanisms and the potential role of alpha-2-adrenoreceptor antagonism in the antipsychotic effects of atypical neuroleptics, particularly considering that co-medication of fluphenazine with the alpha-2-adrenoreceptor antagonist idazoxan enhanced its antipsychotic and cognitive effectiveness (Litman et al., 1996). Our results complement these observations, highlighting the importance of adrenoreceptors as targets for treating cognitive difficulties like those experienced by patients with schizophrenia (McAllister, 2001; Masana et al., 2011).[3] |
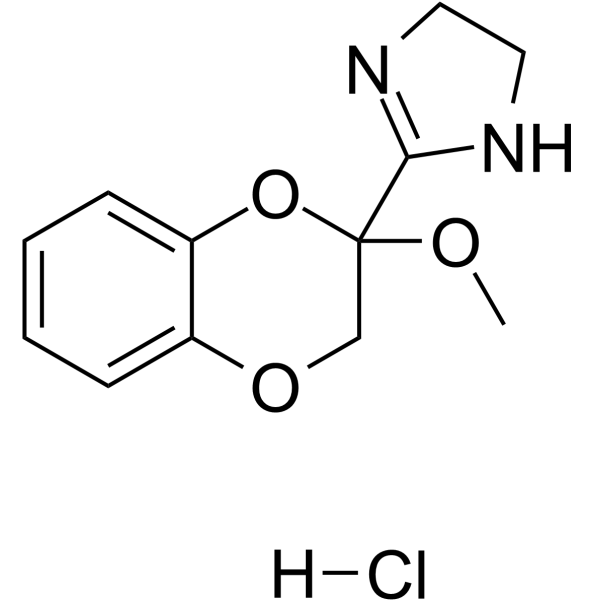
| 分子式 |
C12H15CLN2O3
|
|---|---|
| 分子量 |
270.71
|
| 精确质量 |
270.077
|
| 元素分析 |
C, 53.24; H, 5.59; Cl, 13.10; N, 10.35; O, 17.73
|
| CAS号 |
109544-45-8
|
| 相关CAS号 |
109544-45-8 (HCl); 102575-24-6
|
| PubChem CID |
11957683
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.368
|
| tPSA |
52.08
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
321
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
IMPOOMVZVWKSAP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C12H14N2O3.ClH/c1-15-12(11-13-6-7-14-11)8-16-9-4-2-3-5-10(9)17-12;/h2-5H,6-8H2,1H3,(H,13,14);1H
|
| 化学名 |
2-(3-methoxy-2H-1,4-benzodioxin-3-yl)-4,5-dihydro-1H-imidazole;hydrochloride
|
| 别名 |
RX 821002 hydrochloride; 109544-45-8; 2-(3-methoxy-2h-1,4-benzodioxin-3-yl)-4,5-dihydro-1h-imidazole,hydrochloride; 2-(2,3-DIHYDRO-2-METHOXY-1,4-BENZODIOXIN-2-YL)-4,5-DIHYDRO-1H-IMIDAZOLE HYDROCHLORIDE; 2-Methoxyidazoxan monohydrochloride; MFCD00069343; 2-(3-methoxy-2H-1,4-benzodioxin-3-yl)-4,5-dihydro-1H-imidazole;hydrochloride; 2-Methoxyidazoxan (monohydrochloride);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: 100 mg/mL (369.40 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 50 mg/mL (184.70 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6940 mL | 18.4699 mL | 36.9399 mL | |
| 5 mM | 0.7388 mL | 3.6940 mL | 7.3880 mL | |
| 10 mM | 0.3694 mL | 1.8470 mL | 3.6940 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。