| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
CSF-1R (IC50 = 0.016 μM); FLT3 (IC50 = 0.39 μM); KIT (IC50 = 0.86 μM); AURKC (IC50 = 1 μM); KDR (IC50 = 1.1 μM)
|
|---|---|
| 体外研究 (In Vitro) |
PLX5622(1–20 μM;3 天)可有效减少小脑切片中的小胶质细胞,同时对少突胶质细胞或星形胶质细胞没有影响。 4 μM 浓度三天后,NG2+ 或 PDGFRα+ 细胞计数减少 30-40%,20 μM 浓度时,该数字上升至 90-95%。尽管小胶质细胞显着减少 (~95%),但暴露于 1 μM 或 2 μM PLX5622 的切片显示 NG2+ 或 PDGFRα+ OPC 没有减少[3]。
无细胞激酶抑制剂谱分析显示,PLX5622 对 CSF1R 具有高度特异性,对两个同源性最高的受体 KIT 和 FLT3 显示出 > 20 倍的选择性(补充表 2, 3)。基于晶体学分析(图 2b;补充表 4),PLX5622 和 PLX3397 之间的两个关键结构差异促成了选择性的提高。首先,PLX5622 中间吡啶环上的 2-氟取代旨在进入 Gly-795 旁边的 CSF1R 独有空间(Gly-795 是通往内部变构口袋的门控残基);而 KIT 和 FLT3 在等效位置都有一个体积更大的半胱氨酸。虽然氢原子和氟原子之间的差异看似很小(氟原子具有稍长的键长和稍大的半径,与氢原子相比,其范德华边缘延伸了约 0.5 Å),但其影响是显著的。PLX5622 两端通过氢键和疏水堆积锚定(图 2b),其氟原子处于固定位置。由于范德华斥力与两个原子之间距离的六次方成正比增加,一个更大的门控残基(如 KIT 和 FLT3 中那样)很可能会因空间位阻而产生能量损失。为了支持这一机制,一个含有与 PLX5622 相同 2-氟取代的 PLX3397 的密切类似物在抑制 CSF1R 方面具有与 PLX3397 相同的效力,但对 KIT 的选择性比 PLX3397 高 10 倍。其次,PLX5622 的末端吡啶基团经过优化,以稳定被置换的近膜域腾出的 CSF1R 变构口袋。当 PLX5622 与 KIT 或 FLT3 结合时,中间吡啶环的位置和方向会导致与门控残基半胱氨酸发生空间冲突,从而损害末端吡啶部分的最佳契合度。[1] CSF1R 激酶抑制剂 PLX5622 和 PLX3397 耗竭小脑切片中的小胶质细胞 [3] 将 PLX5622 或 PLX3397 以 1 至 20 μM 的递增浓度应用于小脑切片。治疗三天后,浓度大于 2 μM 的 PLX5622 和 PLX3397 均消除了超过 95% 的小胶质细胞(图 1A,B)。在 1 μM 浓度下,PLX5622 比 PLX3397 多耗竭 15%(Iba1+ 细胞损失:94.6±1.1% vs 79.9±2.4%)(图 1A,B)。在 2 μM 浓度下小胶质细胞损失超过 95% 时,通过 PLP-eGFP 表达或 GFAP 染色评估,未观察到少突胶质细胞或星形胶质细胞的活力或形态发生任何变化(图 1C)。 高浓度的 PLX5622 和 PLX3397 在离体和小脑切片中对 OPCs 具有细胞毒性 [3] 由于 CSF1R 抑制剂可能结合多种酪氨酸激酶,我们检测了 PLX5622 和 PLX3397 对依赖酪氨酸激酶 PDGFRα 存活的 OPCs 的影响。将小脑外植体暴露于递增的 PLX 浓度 3 天后,通过 NG2 或 PDGFRα 免疫染色评估 OPC 细胞数量。在 4 μM 浓度下,PLX5622 导致 NG2+ 或 PDGFRα+ 细胞减少 30-40%;在 20 μM 浓度下,这一减少增加到 90-95%。尽管小胶质细胞被强力耗竭(约 95%),但在暴露于 1 μM 或 2 μM PLX5622 的切片中未观察到 NG2+ 或 PDGFRα+ OPCs 的减少(图 2A,C)。相比之下,用 PLX3397 处理在所有测试浓度下均显著减少 OPC 数量。我们在 0.5 μM 浓度下观察到 NG2+ 或 PDGFRα+ 细胞损失 30-35%,尽管小胶质细胞耗竭不完全(约 70%)。PLX3397 导致的 OPC 损失呈浓度依赖性,在 1 μM 浓度下减少 75-85%,在 2 μM 和 20 μM 浓度下损失 > 90%(图 2B,D)。 在纯化的原代小鼠 OPC 培养物中也观察到对 OPCs 的类似效应。细胞类型特异性标记物染色显示,培养物由 >92% 的 OPCs(PDGFRα)、<1% 的星形胶质细胞(GFAP)和 3.7±2.1% 的小胶质细胞(Iba1)组成(补充图 S1A)。大多数少突胶质细胞是 OPCs,但偶尔(<0.5%)观察到成熟的 OLs(O4+,具有高度复杂的突起网络)(数据未显示)。低浓度(0.5 μM)的 PLX5622 在 24 小时后对活力没有影响;然而,20 μM PLX5622 导致 OPC 死亡增加。相比之下,PLX3397 处理在 0.5 μM 和 20 μM 浓度下均引起显著的细胞毒性(补充图 S1B,C)。这些结果表明,PLX CSF1R 抑制剂能够以浓度依赖的方式直接损害 OPC 的活力。 为了研究长期小胶质细胞耗竭对 OPC 存活的影响,将 2 μM PLX5622 应用于小脑切片 8 天。尽管小胶质细胞被完全清除,但与 DMSO 处理的对照组相比,我们观察到 NG2+ 细胞数量没有差异。此外,长期暴露于 PLX5622 对少突胶质细胞(通过 PLP-eGFP 评估)和星形胶质细胞(通过 GFAP 和 S100b 评估)的形态或数量没有影响(图 3A,B 和数据未显示)。髓鞘蛋白表达(通过 PLP 免疫荧光测量)没有变化(图 3A)。 综上所述,离体和小脑切片数据均表明,低浓度(≤ 2 μM)的 PLX5622 能在不直接影响 OPCs、少突胶质细胞或星形胶质细胞的情况下完全耗竭小胶质细胞。即使在低浓度下,PLX3397 也会导致存活的 OPCs 显著减少。在较高浓度下,两种 PLX 化合物在原代细胞培养物和小脑切片中都会减少 OPC 细胞数量。 在小脑切片中,PLX5622(1–20 μM;3 天)半富马酸盐可有效消耗小胶质细胞,同时对少突胶质细胞或星形胶质细胞没有影响。当暴露于 PLX5622(4 μM;3 天)半富马酸盐时,NG2+ 或 PDGFRα+ 细胞减少 30-40%;在 20 μM 时,这一比例上升至 90-95%。尽管小胶质细胞明显减少 (~95%),但经 1 μM 或 2 μM PLX5622 处理的切片显示 NG2+ 或 PDGFRα+ OPC 没有变化[3]。 |
| 体内研究 (In Vivo) |
PLX5622(1200 ppm;饲料;持续 3 周或 3 天;成年 C57/Bl6 野生型小鼠)在治疗 3 天和 3 周后分别导致约 80% 和 99% 的小胶质细胞丢失。 PLX5622 中皮质、纹状体、小脑和海马中的小胶质细胞减少(成年 C57/Bl6 野生型小鼠,3 个月大;喂养 3 周)[4]。
在总共 14 天中,PLX5622 ( 50 mg/kg;腹腔注射;新生大鼠每日一次,成年大鼠每日两次)可使小胶质细胞在 3 天内减少 80-90%,在 7 天内减少 90% 以上。 PLX5622 治疗 14 天后,成人和新生儿的小胶质细胞减少 > 96%,同时维持基线星形胶质细胞数量。 (消除新生儿的小胶质细胞只需每天注射一次悬浮在 5% 二甲亚砜中的 0.65% PLX5622 和 0.01 M PBS 中的 20% Kolliphor RH40;成人消除则需要每天注射两次。)[5]. PLX5622 (在 AIN-76A 标准饲料中配制,浓度为 1200 毫克/千克;持续 28 天)减少 14 个月大的 5xfAD 小鼠中整个 CNS 的小胶质细胞[6]。 许多阿尔茨海默病(Alzheimer's disease, AD)发展的风险基因在髓系细胞中独家或高度表达。小胶质细胞(Microglia)的生存依赖于集落刺激因子1受体(colony-stimulating factor 1 receptor, CSF1R)信号传导。我们设计并合成了一种高选择性、可穿透血脑屏障的CSF1R抑制剂(PLX5622),能够在病理发展之前和期间实现长期且特异性的小胶质细胞清除。我们发现,在AD的5xFAD小鼠模型中,小胶质细胞清除后,实质空间内无法形成斑块,除了含有存活小胶质细胞的区域。相反,Aβ沉积在皮质血管中,让人联想到脑淀粉样血管病(cerebral amyloid angiopathy)。5xFAD海马体中改变的基因表达也因小胶质细胞的缺失而逆转。对残余斑块形成小胶质细胞的转录组分析显示,它们表现出疾病相关小胶质细胞(disease-associated microglia)的特征。总之,我们描述了PLX5622的结构、配方和功效,它能够实现持续的小胶质细胞清除,并确定了小胶质细胞在启动斑块发病机制中的作用。[1] 引言 神经病理性疼痛是一种使人衰弱的疾病。神经免疫相互作用在神经病理性疼痛中的重要性已通过不同免疫细胞参与病理性疼痛的外周和中枢敏化得到证实。巨噬细胞和小胶质细胞分别是受损神经和脊髓中激活的最丰富的免疫细胞。多条证据表明,巨噬细胞/小胶质细胞的存活、激活、增殖和分化需要巨噬细胞集落刺激因子(macrophage-colony stimulating factor)的参与。在本研究中,我们研究了阻断巨噬细胞集落刺激因子/集落刺激因子1受体(macrophage-colony stimulating factor/colony stimulating factor 1 receptor)信号传导是否能有效缓解神经病理性疼痛。材料与方法 在小鼠中进行部分坐骨神经结扎(Partial sciatic nerve ligation)以诱导神经病理性疼痛行为。在预防性方案(手术前两天开始直至部分坐骨神经结扎后第14天)和逆转性方案(部分坐骨神经结扎后第28天至第33天)中,每日口服给予选择性集落刺激因子1受体抑制剂 PLX5622。使用冯弗雷细丝(von Frey hairs)和丙酮涂抹(acetone application)监测动物的神经病理性疼痛行为。在损伤后第3天和第33天,使用流式细胞术分析受损神经中巨噬细胞的表型。使用Iba-1抗体通过免疫组织化学进一步检测PLX5622对腰段脊髓中小胶质细胞活化的影响。结果 在预防性和逆转性方案中,均观察到PLX5622治疗的动物其机械性痛觉超敏(mechanical allodynia)和冷诱发性痛觉超敏(cold allodynia)显著减轻。PLX5622治疗减少了受损神经中巨噬细胞的总数,似乎集落刺激因子1受体抑制更特异地影响了CD86+(M1样)巨噬细胞。因此,各种促炎细胞因子(TNF-α, IL-1β)的表达降低。在预防性和逆转性方案中,PLX5622治疗显著抑制了部分坐骨神经结扎后腰段脊髓背角(dorsal horn)的小胶质细胞活化。结论 外周神经中的巨噬细胞和脊髓中的小胶质细胞是损伤相关神经病理性疼痛的产生和维持所必需的。阻断疼痛传导通路中这些髓系细胞上的巨噬细胞集落刺激因子/集落刺激因子1受体信号传导是缓解神经病理性疼痛的有效策略。[2] 引言 神经病理性疼痛是一种使人衰弱的疾病。神经免疫相互作用在神经病理性疼痛中的重要性已通过不同免疫细胞参与病理性疼痛的外周和中枢敏化得到证实。巨噬细胞和小胶质细胞分别是受损神经和脊髓中激活的最丰富的免疫细胞。多条证据表明,巨噬细胞/小胶质细胞的存活、激活、增殖和分化需要巨噬细胞集落刺激因子的参与。在本研究中,我们研究了阻断巨噬细胞集落刺激因子/集落刺激因子1受体信号传导是否能有效缓解神经病理性疼痛。材料与方法 在小鼠中进行部分坐骨神经结扎以诱导神经病理性疼痛行为。在预防性方案(手术前两天开始直至部分坐骨神经结扎后第14天)和逆转性方案(部分坐骨神经结扎后第28天至第33天)中,每日口服给予选择性集落刺激因子1受体抑制剂 **PLX5622**。使用冯弗雷细丝和丙酮涂抹监测动物的神经病理性疼痛行为。在损伤后第3天和第33天,使用流式细胞术分析受损神经中巨噬细胞的表型。使用Iba-1抗体通过免疫组织化学进一步检测PLX5622对腰段脊髓中小胶质细胞活化的影响。结果 在预防性和逆转性方案中,均观察到PLX5622治疗的动物其机械性痛觉超敏和冷诱发性痛觉超敏显著减轻。PLX5622治疗减少了受损神经中巨噬细胞的总数,似乎集落刺激因子1受体抑制更特异地影响了CD86+(M1样)巨噬细胞。因此,各种促炎细胞因子(TNF-α, IL-1β)的表达降低。在预防性和逆转性方案中,PLX5622治疗显著抑制了部分坐骨神经结扎后腰段脊髓背角的小胶质细胞活化。结论 外周神经中的巨噬细胞和脊髓中的小胶质细胞是损伤相关神经病理性疼痛的产生和维持所必需的。阻断疼痛传导通路中这些髓系细胞上的巨噬细胞集落刺激因子/集落刺激因子1受体信号传导是缓解神经病理性疼痛的有效策略。[3] 使用特异性CSF1R抑制剂 **PLX5622** 清除老年5xFAD小鼠中的小胶质细胞 PLX5622是一种可穿透血脑屏障的CSF1R抑制剂,能快速清除小胶质细胞,但不抑制c-kit(Valdearcos et al., 2014; Dagher et al., 2015)。为了确认PLX3397的效果并排除其他脱靶效应,我们用含1200 mg/kg PLX5622的饲料处理第二组5xFAD小鼠28天。我们再次使用硫磺素-S(Thioflavin-S)染色组织以显示致密核心斑块(dense core plaques),并用IBA1对微胶质细胞进行免疫标记(图5A)。与PLX3397治疗一样,经PLX5622治疗后小胶质细胞被显著清除,剩余细胞大多与致密核心斑块相关(图5B和E)。量化显示平均每个斑块约有一个剩余细胞(图5C);然而,许多斑块没有相关的IBA1+细胞(图5D)。进一步分析显示,所有剩余的IBA1+细胞的胞体都含有β-淀粉样蛋白(amyloid-β)(图5K);相比之下,未经治疗的5xFAD大脑斑块周围的大多数IBA1+细胞不含有任何β-淀粉样蛋白(图5J)。事实上,在治疗组小鼠中,其中一些IBA1+细胞呈硫磺素-S阳性(图5E)。然而,与PLX3397治疗的小鼠一样,在这组小胶质细胞被清除的5xFAD小鼠中,我们未观察到斑块面积或总数发生变化(图5F和G),β-淀粉样蛋白水平也无显著变化(图5H和I)。[6] PLX5622 半富马酸盐在临床前研究中的药效学 在成年 C57/Bl6 野生型小鼠中,PLX5622(1200 ppm;喂食;持续 3 周或 3 天)半富马酸盐在治疗 3 天后分别导致约 80% 和 99% 的小胶质细胞被破坏。 PLX5622(成年 C57/Bl6 野生型小鼠,3 个月大;喂养 3 周)中大脑、纹状体、小脑和海马中的小胶质细胞减少[4]。半反丁烯二酸盐(PLX5622;50 mg/kg;腹腔注射;新生大鼠每天一次,成年大鼠每天两次)在治疗 3 天期间使小胶质细胞减少 80-90%,到 7 天时,已增加至 > 90% 。 PLX5622 治疗 14 天后,新生儿和成人的小胶质细胞减少了 96% 以上,同时星形胶质细胞的数量保持不变。 (对于新生儿小胶质细胞消除,每天注射一次悬浮在 5% 二甲亚砜中的 0.65% PLX5622 和 0.01 M PBS 中的 20% Kolliphor RH40 就足够了;成人消除需要每天注射两次)[5]。在 14 个月大的 5xfAD 小鼠中,PLX5622(在 AIN-76A 正常饲料中配制,浓度为 1200 mg/kg;持续 28 天)半富马酸盐可减少整个中枢神经系统的小胶质细胞[6]。 PLX5622半富马酸盐在临床前物种中的药代动力学[4] 物种 IV PO (灌胃) 剂量 (mg/kg) AUC0-∞ (ng·hr/mL) CL (mL/min/kg) Vss (L/kg) t1/2 ( hr ) 剂量 (mg/kg) AUC0-∞ (ng·hr/mL) Cmax (ng/mL) F 小鼠 1.92 15,500 2.1 0.34 2.6 45 215,000 26,300 59% 大鼠(雄性) 1.13 2,630 7.7 1.2 2.3 45 99,600 12,000 9 5 %大鼠(雌性) 1.13 5,110 3.7 1.0 3.9 45 181,000 15,600 89% 狗 1.00 6,230 3.0 2.3 15 45 96,500 3,630 34% 猴 1.35 2,100 11 1.6 2.2 ND ND ND ND PLX5622 半富马酸盐灌胃给药悬浮液的制备[4] PLX5622 半富马酸盐是以最终剂量溶液 20 倍的浓度溶解在 DMSO 中。复合原料具有轻度保护作用。每周都会创建新库存。由于其溶解缓慢,稀释剂的成分通常提前一天或更长时间准备: a) 2%羟丙基甲基纤维素(HPMC):将2.0g粉末添加到100mL去离子水中; b) 25% 聚山梨醇酯 80 (PS80):将 25 g 粉末添加到 100 mL 去离子水中。将 4 mL 25% PS80 库存(1% 最终)和 25 mL 2% HPMC 库存(0.5% 最终)添加到 71 mL 去离子水中,制成最终 100 mL 的稀释剂。化合物混合后,最终成分为 0.5% HPMC、1% PS80 和 5% DMSO。在每个剂量日,化合物储备液以这种方式稀释 20 倍:将 19 体积的稀释剂量入管中,并添加 1 体积的化合物/DMSO 储备液 (20x)。为了产生均匀的悬浮液,通过倒置将管中的内容物混合,然后在关闭盖子后将其放入超声水浴中。 |
| 细胞实验 |
小脑切片培养 [3]
矢状小脑切片(300微米)从出生后10-12天(P10-12)的PLP-eGFP小鼠(Mallon等人,2002)制备,并在MilliCell 0.4微米膜插入式培养皿中,使用切片培养基于37°C培养(Sheridan和Dev,2012)。切片培养基成分为:25% Hank氏平衡盐溶液(HBSS),25%热灭活马血清,50%最低必需培养基(MEM),125mM HEPES,28mM D-葡萄糖,2mM L-谷氨酰胺,10单位/毫升青霉素/链霉素。铺板后24小时内更换培养基,之后每2-3天更换一次。切片在处理前培养7-10天。DMSO、PLX5622和PLX3397按指定浓度加入切片培养基中,处理3天或8天。培养基每2-3天更换一次。处理后,切片在PBS中漂洗一次,在4%多聚甲醛(溶于PBS)中固定30分钟,然后进行免疫染色。 纯化原代OPC细胞培养 [3] 原代小鼠OPC培养物按照所述方法(Liu等人,2016)从PLP-eGFP幼鼠制备。简言之,从出生后0-1天(P0-1)小鼠的分离皮层制备混合胶质细胞培养物,并铺板在聚-D-赖氨酸包被的T75培养瓶上。通过将长满的混合胶质细胞培养瓶在200转/分下震荡过夜以分离OPCs,从而制备纯化的OPC单层培养物。OPCs铺板在聚-D-赖氨酸/层粘连蛋白包被的培养皿上,并在含10纳克/毫升PDGF(血小板衍生生长因子)/FGF(成纤维细胞生长因子)的培养基中维持24-48小时,然后进行实验。 IncuCyte活细胞成像 [3] 活细胞成像按所述方法进行(Liu等人,2016)。细胞在24孔板中于细胞培养箱内生长并进行扫描。使用10倍物镜,在每个孔中随机选择9个位置,以15分钟为间隔,使用高清相差显微术和落射荧光显微术(使用585/635纳米滤光片,红色荧光,用于检测DRAQ7死细胞核)进行扫描。图像处理和细胞计数使用IncuCyte和ImageJ软件进行。 |
| 动物实验 |
Drug preparation and treatment [2]
PLX5622 is a potent inhibitor of CSF1R tyrosine kinase activity (KI = 5.9 nM) with at least 50-fold selectivity over four related kinases and over 100-fold selectivity against a panel of 230 kinases. The molecule has been used to investigate the critical role of microglia/macrophages in many different circumstances. The dose in the current study was suggested by the company and in accordance with these previous reports where 1200 ppm in chow daily is sufficient to eliminate microglia fully or 300 ppm in chow daily can reduce partially microglia. Our 65 mg/kg dose nominally is close to the 300 ppm chow daily dose which allows us to lower macrophages in nerves. The drug was prepared following manufacturer’s protocol. Briefly, PLX5622 stock was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 130 mg/ml; 2% hydroxypropyl methyl cellulose and 25% polysorbate 80 were prepared to make a diluent. On each dosing day, PLX5622 stock was diluted 20-fold by adding 1 volume of drug stock (130 mg/ml) in 19 volumes of diluent, making a working solution at 6.5 mg/ml. Vehicle solution was prepared with a mix of diluent and DMSO. Mice were treated daily by oral gavage with 100 μl solution (6.5 mg/ml) per 10g body weight (final dose at 65 mg/kg body weight). To study preventive effect of PLX5622, mice were treated with either a drug or vehicle two days prior to surgery, then daily until D14 post-PSNL. For the reversal effect of PLX5622, treatment started at D28, daily until D33 post-PSNL. Behavioral analysis [2] Mice were habituated to the testing environment 1 to 2 h daily for at least two days before baseline testing. Mice were treated with either vehicle or PLX5622 daily in the morning. The investigator was blinded to the treatment conditions. Animal Chow Treatment [3] Male and female PLP-eGFP animals (4-5 per group) aged 8-12 weeks were placed on formulated PLX Chow (1200 mg/kg for PLX5622 and 275 mg/kg for PLX3397) or standard diet for 7 or 21 days. Following treatment, animals were perfused with ice cold PBS followed by 4% paraformaldehyde in PBS. Brains and spinal cords were dissected, post-fixed overnight and then cryoprotected. Tissues were sectioned at 30um on a cryostat and collected in PBS for free-floating immunostaining. For brain-wide microglia ablation, adult C57/Bl6 wild type mice between 8-16 weeks of age were treated with CSF1R inhibitor, PLX5622, or control chow (same formula lacking only the inhibitor) for 3 weeks or 3 days as indicated. While a 3-week long PLX5622 treatment leads to a 99% microglia loss, around 80% of microglia are already lost after 3 days [4]. Habituation to head-fixation and intraperitoneal (i.p.) injection. [4] After a 1-2 week recovery from the surgery, the mice were randomly divided into 2 groups, one group received PLX5622 and one group received control chow (lacking inhibitor). The mice were later put under water restriction (1.5 ml/day) and were handled and habituated daily to head-fixation and immobilization for ~2 weeks. They were immobilized in a polyethylene tube and head-fixed in the future recording environment under 2-photon microscope. During this habituation period, we increased the head-fixation period from 3 minutes to 40 minutes gradually. After they showed no signs of stress and drank water provided randomly during the 40-minute session, we switched to no-water-provided head fixation habituation for future recording. After habituation to head-fixation, we also performed daily habituation to i.p. injection before the head-fixation session. Mice were injected i.p. with a microlitre volume equivalent to 10× the body weight in grams (10 × BW μl) of saline, matching the volume to be injected in future imaging sessions, and then performed head-fixation. This habituation was performed for ~1 week until the mice showed a reduction in clear signs of stress upon handling and i.p. injection. Microdialysis [4] C57/Bl6 male mice were put on PLX5622 or control chow (n=5 PLX5622 diet, n=5 control diet). for one week. Dialysis probes were implanted and mice recovered for one week before commencing microdialysis collection. Microdialysis guide cannulae were stereotaxically implanted in the striatum (A/P: +1.4 mm; M/L: −1.0 mm; D/V: −3.8 mm from skull). Microdialysis experiments were conducted following a one-week recovery period following guide cannula implantation. Dialysis tubing was flushed prior to initial use with 70% EtOH for 5 minutes, followed by dH2O syringe pump at a flow rate of 1 μL/min. The tubing was then attached to the microdialysis probe (Cuprophane (6kD), membrane length 1mm), which was primed by placing the probe in artificial cerebrospinal fluid (aCSF; pH 7.4: 148 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2) and running aCSF through the microdialysis tubing and probe at a 0.8 μL/min rate. Microdialysis experiments were done in anesthetized animals. Briefly, mice were placed into a stereotaxic frame under isoflurane anesthesia (4% induction,1.75% sustained). The probe was inserted via the guide cannula and allowed to equilibrate. Dialysate was collected after 20 minutes. All collections were frozen at −80 degrees Celsius immediately following collection completion. Microglia inhibition via intraperitoneal injection of PLX5622 [5] Prior CSF1R inhibitor studies using PLX3397/5622-integrated chow treated animals for at least 1 week prior to their experimental manipulation (e.g. Elmore et al., 2014; Okunukia et al., 2019). To ensure adequate microglia depletion for neonatal surgeries, we began our treatments at P1, with an equivalent pre-surgery treatment duration (9 days) for adults. Pilot testing indicated that while a single daily injection of 0.65% PLX5622 suspended in 5% dimethyl sulfoxide and 20% Kolliphor RH40 in 0.01 M PBS was sufficient for neonatal microglia depletion, adult depletion required injections twice daily (10–12 h apart). Starting at P1 (for P10 CTX) or P41 (for P50 CTX), additional rats were injected with either PLX5622 (50 mg/kg; n = 4–5/condition) or a vehicle solution (n = 3–4/condition) once (neonates) or twice (adult) a day until being sacrificed 4 days after surgery. Compounds [6] PLX3397 was formulated in AIN-76A standard chow by Research Diets Inc. at 290 mg/kg or 600 mg/kg, as previously described (Elmore et al., 2014). PLX5622 was provided by Plexxikon Inc. and formulated in AIN-76A standard chow at 1200 mg/kg. Animal treatments [6] Crossing these mice yielded CSF1R-iCRE/Rosa26YFP progeny that express yellow fluorescent protein (YFP) in all cells that either transiently or constitutively express CSF1R, which in the brain predominantly labels microglia. Two-month-old mice were treated for 7 days with PLX3397 (600 mg/kg in chow) to eliminate microglia. The 5xfAD mouse model has been previously described in detail (Oakley et al., 2006). Using a 2 × 2 factorial design, forty male and female 10-month-old wild-type (C57BL/6 background) or 5xfAD mice were treated with either PLX3397 for 28 days to eliminate microglia or control chow, creating four treatment groups (n = 10/group): Control (six males and four females), PLX3397 (six males and four females), 5xfAD (four males and six females), and 5xfAD + PLX3397 (four males and six females). At this age, 5xfAD mice display extensive pathology, synaptic loss, and neuronal loss (Oakley et al., 2006; Buskila et al., 2013; Eimer and Vassar, 2013). After 28 days of treatment, behavioural testing commenced while animals remained on their respective diets. A second cohort of 14-month-old 5xfAD mice (n = 4/group; 5xfAD = four males, and 5xfAD + PLX5622 = three males and one female) was treated with either PLX5622 for 28 days to deplete microglia or control chow (5xfAD versus 5xfAD + PLX5622). A third cohort of 1.5-month-old 5xfAD mice (n = 4/group; two males and two females) was treated with PLX3397 or control for 28 days (5xfAD versus 5xfAD + PLX3397). Following behavioural testing in the 10-month-old cohort, and following inhibitor treatment in the 14-month-old and 1.5-month-old cohorts, mice were euthanized via CO2 inhalation and transcardially perfused with phosphate-buffered saline. For both studies, brains were removed and hemispheres separated along the midline. Brain halves were either flash frozen for subsequent biochemical analysis, drop-fixed in 4% paraformaldehyde for subsequent immunohistochemical analysis, or placed in Golgi impregnation solution for subsequent dendritic spine analysis. Fixed half brains were sliced at 40 µm using a Leica SM2000 R freezing microtome. The flash-frozen hemispheres were ground with a mortar and pestle to yield a fine powder. One-half of the powder was homogenized in Tissue Protein Extraction Reagent, T-PER with protease and phosphatase inhibitors. The second half was processed with an RNA Plus Universal Mini Kit for RNA analysis. |
| 药代性质 (ADME/PK) |
In vivo PLX5622 demonstrated desirable PK properties in mice, rats, dogs, and monkeys (Supplementary Table 5), with a brain penetrance of ~20% (compared to ~5% for PLX339717; Supplementary Table 6). The improved blood-brain barrier (BBB) penetrance of PLX5622 over PLX3397 is consistent with the physicochemical properties of the two compounds (Supplementary Table 7). PLX5622 has lower molecular weight, higher lipophilicity and better cell permeability, all factors known to influence the ability of a compound to cross the BBB. PLX5622 was formulated in rodent chow, and administration to mice showed highly effective, causing a 90% reduction with 1200 ppm chow within 5 days of treatment (Fig. 2d, e). Instructions for the synthesis and formulation of PLX5622 are provided (Supplementary Methods). [1]
|
| 参考文献 | |
| 其他信息 |
PLX5622 is a highly selective brain penetrant and orally active CSF1R inhibitor. PLX5622 allows for extended and specific microglial cells elimination, preceding and during pathology development. PLX5622 demonstrates desirable PK properties in varies animals. PLX5622 is mostly used in the way of feed free diet.
In conclusion, we have designed and created a specific CSF1R inhibitor, PLX5622, that allows for the sustained and specific elimination of microglia. This novel method of microglial depletion provided us with the means to eliminate microglia for the duration of AD pathogenesis. Ultimately, these data demonstrate that microglial elimination is associated with the prevention of plaque formation and the downregulation of hippocampal neuronal genes that occur in a preclinical model of AD progression. These results indicate that microglia appear to contribute to multiple facets of AD etiology – microglia appear crucial to the initial appearance and structure of plaques, and following plaque formation, promote a chronic inflammatory state modulating neuronal gene expression changes in response to Aβ/AD pathology. [1] Synergistic blocking spinal microglia and nerve macrophages activation could result in significant alleviation of neuropathic pain behaviors, which was evidenced by the effect of CSF1R inhibitor in our study. Peripheral macrophage activation, more specifically inflammatory macrophages expressing CD86, TNF-α, or IL-1β in injured peripheral nerves, is prominently affected by CSF1R inhibition while CD206 expressing macrophages were not significantly altered. Moreover, PLX5622 treatment effectively reduced microglia activation in spinal cord following nerve injury. Although our results provided a proof of concept on the critical role of microglia/macrophages in neuropathic pain, and that CSF1R could be an interesting target for neuropathic pain alleviation, it is however important to bear in mind that blocking M-CSF/CSF1R signaling, especially when the drug is given systemically, it affects not only microglia/macrophage in the nervous system, myeloid cells in other tissues could be most likely affected. Thus, to promote the translation of the current finding, not only more evidence from other animal models of chronic pain are awaited but also thorough analysis of the properties/safety of the compound in other organs is required.[2] In summary, by comparing the effect of PLX5622 and PLX3397, we confirm that CSF1R inhibitors can efficiently deplete microglia ex vivo and in vivo without affecting mature oligodendrocytes or myelin protein expression. In addition, we speculate that CSF1R inhibitors may directly impact OPC viability through off-target binding to PDGFRα, a member of the type III tyrosine kinase receptor family. Therefore, inhibitor concentration and duration of treatment must be carefully adjusted for the relevant experimental system. These data also question whether microglia are essential for OPC viability in ex vivo slice cultures and adult brain.[3] In summary, we demonstrated glial responses to CTX that varied based on developmental stage. Adult CTX induces large microglia and small astrocyte responses, but CTX at P10 results in small microglia and large astrocyte responses, which are associated with differences in CTX recovery (Kopka et al., 2000; Martin et al., 2019; Reddaway et al., 2012; Sollars, 2005; St. John et al., 1995). We are the first to demonstrate that i.p. administration of PLX5622 to both early postnatal and adult rats depletes microglia without impacting animal health (as assessed by weight) or astrocyte quantity. While such depletion eliminated the adult astrocyte response, the neonatal response appeared unaffected, showing that microglia are likely not required for neonatal CTX-induced astrogliosis. Our findings suggest the presence of pathway-specific developmental differences which may contribute to differences in recovery across development. Our results add to existing knowledge regarding differences in glia function across development and factors that may contribute to why the developing gustatory system has difficulty recovering from injury. Our findings highlight the potential importance of central glia to both the developing and injured gustatory system and suggest differences in reciprocal interactions between microglia and astrocytes across maturational stages.[5] We have previously shown that microglial elimination can be achieved in the healthy adult mouse brain by treatment with small-molecule inhibitors of CSF1R (Elmore et al., 2014; Dagher et al., 2015), with many groups confirming our findings (Valdearcos et al., 2014; Asai et al., 2015; Klein et al., 2015; Schreiner et al., 2015). Here, we have extended these findings in mice constitutively expressing YFP under the Rosa26 locus in all CSF1R expressing cells to definitively show that microglia are eliminated and are not simply downregulating myeloid/microglial markers. We also found that chronically activated microglia following extensive neuronal injury can be eliminated with the same approach. Our studies also revealed that microglial elimination following neuronal insult improved functional outcomes, whereas elimination of microglia during the lesion exacerbated neuronal loss, revealing differential roles of microglia in injury response (Rice et al., 2015). It is important to note that only blood–brain barrier-permeant CSF1R inhibitors are able to eliminate microglia. These include the compounds PLX3397 and PLX5622 used in this study, as well as BLZ945 (Pyonteck et al., 2013). Furthermore, sustained brain exposure levels of CSF1R inhibitors are required for effective microglial elimination. It takes at least 3 days for microglia to succumb and die within the CNS (Elmore et al., 2014). It should be noted that CSF1R inhibitors are highly versatile; at high doses they eliminate microglia, allowing for the exploration into the roles of these cells in disease and normal brain function, while at lower doses they modulate CSF1R signalling in microglia without eliminating them. To this end, we have previously used low doses of PLX5622 (300 mg/kg chow rather than the 1200 mg/kg chow used in this study) to explore the role of the CSF1R in microglial response to Alzheimer’s disease pathology and found that it regulates the chemotactic response of these cells to plaques (Dagher et al., 2015), resulting in improvements in cognition without affecting plaque burden or inflammatory profile. Moreover, the CSF1R has also been shown to be crucial for microglial proliferation during disease (Gomez-Nicola et al., 2013), also using CSF1R inhibitor paradigms that do not result in microglial elimination. In contrast, in this study, we sought to define the roles of microglia in mediating Alzheimer’s disease pathogenesis through the administration of CSF1R inhibitors at doses sufficient to eliminate microglia, rather than at lower doses that modulate microglial function without eliminating them. We first set out to determine if microglia in the brains of aged 5xfAD mice are still dependent on CSF1R signalling for their survival. Importantly, we show that 28 days of continuous treatment with either PLX3397 or PLX5622 leads to an ∼80–90% reduction in microglia, respectively, throughout the CNS in adult 5xfAD mice. [6] |
| 分子式 |
C23H21F2N5O3
|
|---|---|
| 分子量 |
453.45
|
| 精确质量 |
395.16
|
| 元素分析 |
C, 60.92; H, 4.67; F, 8.38; N, 15.44; O, 10.58
|
| 相关CAS号 |
PLX5622;1303420-67-8; 2743279-01-6 (HCl); 2749102-07-4 (fumarate)
|
| PubChem CID |
154731123
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| tPSA |
226 Ų
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
18
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
66
|
| 分子复杂度/Complexity |
648
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC1=CC2=C(NC=C2CC3=C(N=C(C=C3)NCC4=C(N=CC(=C4)F)OC)F)N=C1.CC1=CC2=C(NC=C2CC3=C(N=C(C=C3)NCC4=C(N=CC(=C4)F)OC)F)N=C1.C(=C/C(=O)O)\C(=O)O
|
| InChi Key |
FMEISBYRQFIJPD-WXXKFALUSA-N
|
| InChi Code |
InChI=1S/2C21H19F2N5O.C4H4O4/c2*1-12-5-17-14(9-26-20(17)25-8-12)6-13-3-4-18(28-19(13)23)24-10-15-7-16(22)11-27-21(15)29-2;5-3(6)1-2-4(7)8/h2*3-5,7-9,11H,6,10H2,1-2H3,(H,24,28)(H,25,26);1-2H,(H,5,6)(H,7,8)/b;;2-1+
|
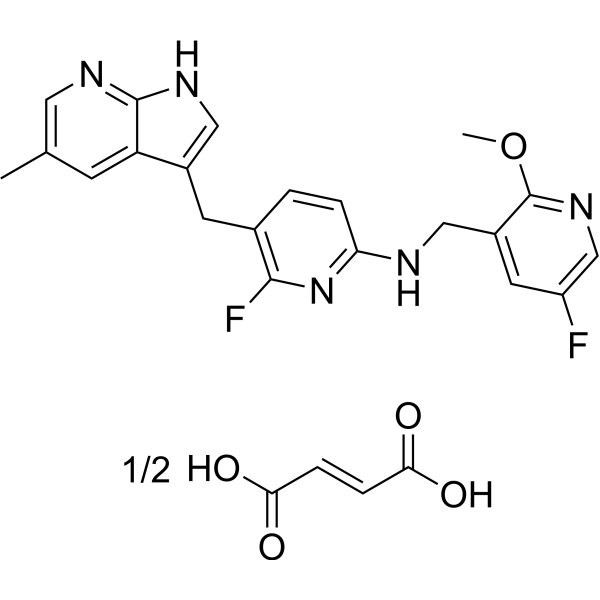
| 化学名 |
(E)-but-2-enedioic acid;6-fluoro-N-[(5-fluoro-2-methoxypyridin-3-yl)methyl]-5-[(5-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]pyridin-2-amine
|
| 别名 |
PLX5622; PLX-5622; PLX5622 (hemifumarate); PLX5622 hemifumarate; PLX 5622; PLX-5622 hemifumarate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO :~100 mg/mL (~220.53 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.25 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 32.5 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.25 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 32.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.25 mg/mL (7.17 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2053 mL | 11.0266 mL | 22.0531 mL | |
| 5 mM | 0.4411 mL | 2.2053 mL | 4.4106 mL | |
| 10 mM | 0.2205 mL | 1.1027 mL | 2.2053 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01282684 | Completed | Drug: PLX5622 Drug: Placebo |
Healthy | Plexxikon | January 2011 | Phase 1 |
| NCT01329991 | Completed | Drug: PLX5622 Drug: Placebo |
Rheumatoid Arthritis | Plexxikon | May 2011 | Phase 1 |