| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
5-HT4A Receptor ( pKi = 8.6 ); 5-HT4B Receptor ( pKi = 8.1 )
Prucalopride Succinate (R-108512, R-93877) is a highly selective agonist of the 5-hydroxytryptamine 4 (5-HT4) receptor, with subtype selectivity: - Human recombinant 5-HT4a receptor: Ki = 1.3 nM (using [³H]-GR113808 as the radioligand) [1] - Human recombinant 5-HT4b receptor: EC50 = 3.8 nM (functional assay measuring cAMP accumulation, full agonism with 95% efficacy vs. 5-HT) [2] - No significant binding to other 5-HT receptor subtypes (5-HT1A/2A/3/7, Ki > 1000 nM) or neurotransmitter transporters (SERT, DAT, NET, Ki > 1000 nM) [1,2] |
|---|---|
| 体外研究 (In Vitro) |
Prucalopride succinate (10 µM; 24, 48, 72 h) 在A549细胞中显示抗增殖活性[4]。 Prucalopride succinate诱导A549/A427细胞自噬和心脏,降低磷酸化蛋白信号B (AKT)和心血管细胞增殖测定细胞系:A549细胞浓度:10μM温育时间:24、48、72h结果:抑制肺癌细胞增殖。
5-HT4受体介导的cAMP蓄积:表达人5-HT4b受体的HEK293细胞经琥珀酸普芦卡必利(0.1–100 nM)处理30分钟,10 nM时细胞内cAMP水平较基线增加85%,EC50=3.8 nM(ELISA检测),最大效能为5-HT(1 μM)的95%[2] - 刺激结肠平滑肌收缩:离体人结肠平滑肌条与琥珀酸普芦卡必利(1–1000 nM)孵育1小时,100 nM时收缩幅度较溶媒组增加60%,EC50=25 nM(力传感器测量)[4] - 对神经元BDNF表达无影响:原代大鼠皮质神经元经琥珀酸普芦卡必利(最高10 μM)处理72小时,BDNF蛋白水平无显著变化(Western blot)[2] |
| 体内研究 (In Vivo) |
普卢卡必利通过刺激近端结肠的高幅度集群收缩和抑制禁食狗远端结肠的收缩活动,以剂量依赖的方式改变结肠收缩运动模式。普芦卡必利还会导致第一次巨大迁移性收缩 (GMC) 时间出现剂量依赖性缩短;使用较高剂量的普卡必利时,第一次 GMC 通常发生在治疗后的前半小时内。
改善洛哌丁胺诱导便秘小鼠的胃肠动力:雄性ICR小鼠(20–25 g)皮下注射洛哌丁胺(10 mg/kg)诱导便秘后,口服琥珀酸普芦卡必利(0.1、0.3、1 mg/kg)。0.3 mg/kg剂量使6小时内粪便颗粒数较溶媒组增加75%,粪便含水量从32%升至48%[3] - 缩短大鼠胃肠转运时间:雄性SD大鼠(250–300 g)口服琥珀酸普芦卡必利(0.05、0.2、0.5 mg/kg),0.2 mg/kg剂量使炭末餐通过胃肠道的时间较溶媒组减少40%(从120分钟降至72分钟)[1] - 无中枢神经系统(CNS)作用:口服琥珀酸普芦卡必利(最高10 mg/kg)的小鼠,旷场实验(运动活性)和高架十字迷宫(焦虑样行为)均无显著变化[2] |
| 酶活实验 |
人5-HT4a受体结合实验:200 μL反应体系包含50 μg表达人5-HT4a受体的HEK293细胞膜蛋白、0.5 nM [³H]-GR113808(放射性配体)及琥珀酸普芦卡必利(0.01–100 nM),在含10 mM MgCl₂的50 mM Tris-HCl(pH 7.4)中25°C孵育60分钟。通过预浸泡0.3%聚乙烯亚胺的玻璃纤维滤膜过滤,冷缓冲液洗涤3次,液体闪烁计数仪检测放射性。非特异性结合(NSB)通过加入10 μM未标记GR113808确定,采用Cheng-Prusoff方程计算Ki[1]
- 5-HT4b受体cAMP功能实验:HEK293-5-HT4b细胞以5×10⁴细胞/孔接种于96孔板,含10% FBS的DMEM培养24小时。培养基更换为含0.5 mM IBMX(磷酸二酯酶抑制剂)的无血清DMEM,加入琥珀酸普芦卡必利(0.1–100 nM),37°C孵育30分钟后用0.1 M HCl裂解细胞。竞争性ELISA检测cAMP水平,从剂量-反应曲线推导EC50[2] |
| 细胞实验 |
离体人结肠平滑肌细胞收缩实验:人结肠平滑肌组织切为2 mm×10 mm条块,悬浮于Krebs-Ringer缓冲液(37°C,95% O₂/5% CO₂)中,平衡1小时后加入琥珀酸普芦卡必利(1–1000 nM)。力传感器连续记录1小时收缩力,数据以1 μM卡巴胆碱诱导的最大收缩为对照归一化[4]
- HEK293-5-HT4b细胞活力实验:表达5-HT4b受体的HEK293细胞以1×10⁴细胞/孔接种于96孔板,经琥珀酸普芦卡必利(0.1–100 μM)处理72小时。加入MTT(5 mg/mL)孵育4小时,DMSO溶解甲瓒结晶,检测570 nm吸光度;各浓度下细胞活力均>95%(相对于溶媒组)[2] |
| 动物实验 |
In the prefrontal cortex, prucalopride maximally increased ACh and histamine levels at 5 and 10 mg/kg, while PRX-03140 significantly increased cortical histamine levels at 50 mg/kg, failing to affect ACh release at doses below 150 mg/kg, according to studies using microdialysis in rats.
Loperamide-Induced Constipation Mouse Model: Male ICR mice (6–8 weeks old, 20–25 g) housed at 22±2°C (12 h light/dark cycle). Randomized into 4 groups (n=10/group): 1. Vehicle: Oral gavage of 0.5% carboxymethylcellulose sodium (CMC-Na, 10 mL/kg); 2. Prucalopride 0.1 mg/kg: Oral gavage of Prucalopride Succinate (0.1 mg/kg, dissolved in 0.5% CMC-Na); 3. Prucalopride 0.3 mg/kg: Oral gavage of Prucalopride Succinate (0.3 mg/kg); 4. Prucalopride 1 mg/kg: Oral gavage of Prucalopride Succinate (1 mg/kg). Mice received loperamide (10 mg/kg, s.c.) 1 hour before drug administration. Fecal pellets were collected for 6 hours to count number and measure water content [3] - Rat Gastrointestinal Transit Model: Male Sprague-Dawley rats (8 weeks old, 250–300 g) fasted for 18 hours, randomized into 4 groups (n=6/group): 1. Vehicle: Oral gavage of 0.5% CMC-Na (10 mL/kg); 2. Prucalopride 0.05 mg/kg: Oral gavage of Prucalopride Succinate (0.05 mg/kg); 3. Prucalopride 0.2 mg/kg: Oral gavage of Prucalopride Succinate (0.2 mg/kg); 4. Prucalopride 0.5 mg/kg: Oral gavage of Prucalopride Succinate (0.5 mg/kg). Thirty minutes after drug administration, rats received a charcoal meal (10% charcoal in 5% gum arabic, 1 mL/100 g, p.o.). Rats euthanized 60 minutes later; gastrointestinal tract dissected. Transit time was calculated as the time for charcoal to reach the cecum [1] |
| 药代性质 (ADME/PK) |
Oral Bioavailability: In male Sprague-Dawley rats, oral Prucalopride Succinate (1 mg/kg) had an oral bioavailability of 82% compared to intravenous administration (0.5 mg/kg) [1]
- Plasma Pharmacokinetics: Rats intravenously injected with 0.5 mg/kg Prucalopride Succinate: Cmax = 0.8 μg/mL, Tmax = 5 minutes, elimination half-life (t1/2) = 3.5 hours. Oral 1 mg/kg: Cmax = 0.6 μg/mL, Tmax = 1.0 hour, t1/2 = 4.2 hours (HPLC-UV detection) [1] - Plasma Protein Binding: Prucalopride Succinate exhibited 30% protein binding in human plasma (ultrafiltration method, plasma concentration range: 0.1–10 μg/mL) [1] - Tissue Distribution: In mice, 1 hour after oral administration of 1 mg/kg Prucalopride Succinate, the highest concentrations were observed in the gastrointestinal tract (colon: 2.8 μg/g) and kidneys (1.5 μg/g); brain/plasma concentration ratio = 0.05 (low CNS penetration) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No published experience exists with prucalopride during breastfeeding. However, the manufacturer reports an unpublished study that indicates a relatively low amount of drug in breastmilk. Until more data become available, monitor the breastfed infant for diarrhea. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Acute In Vivo Toxicity: The LD50 of Prucalopride Succinate in male ICR mice (intraperitoneal injection) was 450 mg/kg. Mice treated with doses >300 mg/kg showed transient diarrhea and lethargy, with no mortality at doses ≤200 mg/kg [1] - Subacute Toxicity: Rats orally administered Prucalopride Succinate (0.1, 1, 10 mg/kg/day) for 28 days showed no significant changes in body weight (<5% variation), serum ALT/AST/BUN/creatinine levels, or histopathological damage in the liver, kidneys, or gastrointestinal tract [1] |
| 参考文献 | |
| 其他信息 |
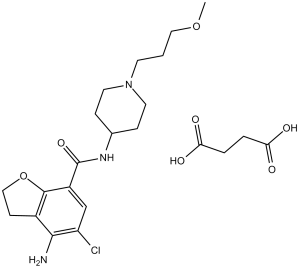
Prucalopride Succinate is the succinate salt form of prucalopride, an orally bioavailable dihydro-benzofuran-carboxamide and selective serotonin (5-HT4) receptor agonist, with gastrointestinal (GI) prokinetic activity. Upon oral administration, prucalopride specifically targets, binds to and stimulates the 5-HT4 receptor. This alters colonic motility patterns and stimulates colonic mass movements. This may normalize bowel movements and may relief chronic constipation. In addition, by increasing esophageal and gastric motility, prucalopride may also provide relief for aspiration-associated symptoms.
See also: Prucalopride (has active moiety). Drug Indication Resolor is indicated for symptomatic treatment of chronic constipation in adults in whom laxatives fail to provide adequate relief. Treatment of chronic constipation, Treatment of opioid-induced constipation Mechanism of Action: Prucalopride Succinate (R-108512, R-93877) exerts prokinetic effects by selectively activating 5-HT4 receptors on gastrointestinal smooth muscle cells and enteric neurons. This activation increases intracellular cAMP levels, enhances smooth muscle contraction, and accelerates gastrointestinal transit [1,4] - Therapeutic Potential: Prucalopride Succinate is clinically approved for the treatment of chronic idiopathic constipation (CIC), particularly in patients unresponsive to laxatives. Preclinical data confirm its ability to reverse drug-induced constipation without CNS side effects [3] - Chemical Properties: Prucalopride Succinate (R-108512, R-93877) is a white crystalline powder soluble in water (25 mg/mL) and DMSO (50 mg/mL). It is stable in aqueous solution (pH 4.0–8.0) for 7 days at room temperature [1] |
| 分子式 |
C22H32CLN3O7
|
|
|---|---|---|
| 分子量 |
485.96
|
|
| 精确质量 |
485.192
|
|
| 元素分析 |
C, 54.38; H, 6.64; Cl, 7.29; N, 8.65; O, 23.05
|
|
| CAS号 |
179474-85-2
|
|
| 相关CAS号 |
Prucalopride; 179474-81-8
|
|
| PubChem CID |
9870009
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
3.117
|
|
| tPSA |
154.91
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
538
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1=C(C2C([H])([H])C([H])([H])OC=2C(=C1[H])C(N([H])C1([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])OC([H])([H])[H])C([H])([H])C1([H])[H])=O)N([H])[H].O([H])C(C([H])([H])C([H])([H])C(=O)O[H])=O
|
|
| InChi Key |
QZRSNVSQLGRAID-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H26ClN3O3.C4H6O4/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14;5-3(6)1-2-4(7)8/h11-12H,2-10,20H2,1H3,(H,21,23);1-2H2,(H,5,6)(H,7,8)
|
|
| 化学名 |
4-amino-5-chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboxamide;butanedioic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0578 mL | 10.2889 mL | 20.5778 mL | |
| 5 mM | 0.4116 mL | 2.0578 mL | 4.1156 mL | |
| 10 mM | 0.2058 mL | 1.0289 mL | 2.0578 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02047045 | Completed | Procedure: acupuncture Drug: Prucalopride |
Constipation | Guang'anmen Hospital of China Academy of Chinese Medical Sciences |
April 2014 | Not Applicable |
| NCT01870674 | Completed | Drug: YH12852 Drug: Prucalopride Drug: Placebo |
Healthy | Yuhan Corporation | August 2013 | Phase 1 |
| NCT03279341 | Completed | Drug: Prucalopride Drug: Bisacodyl Drug: polyethylene glycol |
Chronic Constipation | University Hospital, Gasthuisberg |
December 3, 2012 | Phase 4 |
| NCT05966246 | Completed | Drug: Arm I : Experimental (Prucalopride succinate group) Drug: Arm II : Control (Mosapride citrate group) |
Gastric Cancer | Gangnam Severance Hospital | January 25, 2022 | Not Applicable |
| NCT01807000 | Completed | Drug: Radiolabeled Prucalopride Succinate |
Healthy | Shire | March 18, 2013 | Phase 1 |
 |
|---|
 |
 |