| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Flt3 (Kd = 1.6±0.7 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:AC220 是一种独特、有效、选择性的 FLT3 抑制剂,对 FLT3 具有高亲和力,Kd 值为 1.6 nM。 AC220 抑制人白血病细胞系 MV4-11 中 FLT3 的自磷酸化,MV4-11 具有纯合 FLT3-ITD 突变且依赖于 FLT3,而 RS4;11 则表达野生型 FLT3,IC50 值分别为 1.1 nM 和 4.2 nM。 AC220 是最有效的细胞 FLT3-ITD 抑制剂,与 IC50 值范围为 0.87 nM 至 64 nM 的所有其他 FLT3 抑制剂相比,对 MV4-11 细胞增殖具有最显着的抑制作用,IC50 为 0.56 nM。 AC220 对 A375 细胞的增殖没有抑制活性,该细胞在 BRAF 中具有激活突变,并且不依赖于 FLT3,这表明 FLT3 抑制和一般细胞毒性作用之间存在很大的窗口。激酶测定:为了测量 FLT3 自磷酸化的抑制,将 MV4-11 或 RS4;11 细胞在低血清培养基 (0.5% FBS) 中培养过夜,并于第二天以每孔 400 000 个细胞的密度接种到 96 孔板中。将细胞与不同浓度的 AC220 在 37°C 下孵育 2 小时。为了在 RS4;11 细胞中诱导 FLT3 自磷酸化,在 AC220 孵育 2 小时后添加 100 ng/mL FLT3 配体 15 分钟。制备细胞裂解物并在预涂有总 FLT3 捕获抗体的 96 孔板中孵育。将包被板与抗 FLT3 的生物素化抗体一起孵育以检测总 FLT3,或与抗磷酸酪氨酸的抗体一起孵育以检测 FLT3 自磷酸化。在这两种情况下,SULFO 标记的链霉亲和素二抗用于在 Meso Scale Discovery 平台上进行电化学发光检测。 AC220抑制FLT3-ITD或TLT3-WT自磷酸化50%的浓度代表IC50值。细胞测定:细胞(MV4-11 和 RS4;11 细胞)在低血清培养基(0.5% FBS)中培养过夜,以每孔 40 000 个细胞接种到 96 孔板中,并在 37° 下暴露于 AC220 72 小时C。使用 Cell Titer-Blue Cell Viability Assay 测量细胞活力。
|
| 体内研究 (In Vivo) |
在 FLT3-ITD 依赖性 MV4-11 肿瘤异种移植小鼠模型中,口服 AC220(10 mg/kg)可诱导 FLT3 自磷酸化的时间依赖性抑制; 2小时时抑制率为90%,24小时时抑制率为40%。 AC220 每天口服一次,剂量低至 1 mg/kg,可显着延长 FLT3-ITD AML 小鼠模型的生存期。使用 10 mg/kg AC220 治疗 28 天,所有小鼠的肿瘤均快速完全消退,并且在治疗后 60 天期间没有肿瘤再生长。与舒尼替尼治疗相比,AC220 显示出更显着的疗效,舒尼替尼治疗使除一只小鼠外的所有小鼠的肿瘤缓慢缩小并在停止治疗后立即恢复生长。
|
| 酶活实验 |
使用 KinomeScan 进行激酶结合实验。 FLT3 测定中使用的激酶构建体仅跨越催化结构域(氨基酸 592 至 969)。该构建体不存在近膜结构域,其目的是量化抑制剂与开放 FLT3 活性位点的内在结合亲和力 [1]。
|
| 细胞实验 |
细胞MV4-11和RS4;11分别在补充有10%胎牛血清(FBS)的Iscove培养基和补充有10%FBS的RPMI中培养。为了进行增殖测定,细胞在低血清培养基(0.5% FBS)中培养整夜后,以每孔 40,000 个细胞的密度接种到 96 孔板中。细胞补充抑制剂(例如 quizartinib)并在 37°C 下孵育 72 小时。 Cell Titer-Blue 细胞活力测定用于测量细胞活力。将细胞在低血清培养基(0.5% FBS)中培养过夜,第二天,将它们以每孔 400 000 个细胞的密度接种到 96 孔板中,以测量 FLT3 自磷酸化的抑制情况。抑制剂(例如 quizartinib)在 37°C 下在细胞中培养两小时。化合物孵育 2 小时后,添加 100 ng/mL FLT3 配体 15 分钟,以引起 RS4;11 细胞中的 FLT3 自磷酸化。将制备的细胞裂解物在预先涂有总 FLT3 捕获抗体的 96 孔板中孵育。使用生物素化的 FLT3 抗体或抗磷酸酪氨酸抗体在包被板上孵育,以检测总 FLT3 或 FLT3 自磷酸化。对于 Meso Scale Discovery 平台上的电化学发光检测,在这两种情况下均使用带有 SULFO 标记的链霉亲和素二抗 [1]。
|
| 动物实验 |
Mice: The mice used are female nu/NU or severe combined immunodeficient mice. Quizartinib (hydrochloride salt) is formulated in 22% hydroxypropyl-β-cyclodextrin, CEP-701 is formulated in 20% gelucire 44/14 in water (vol/vol), MLN-518 and SU 11248 are formulated in 10 mM sodium citrate (pH 3.5), PKC-412 is formulated in 3:1 gelucire 44/14-propylene glycol (vol/vol), and Bay 43-9006 is formulated in 80% PEG-400. Compound concentrations are selected in a volume of 10 mL/kg to deliver the intended dose. Oral gavage is used to administer compounds, and plasma samples are taken 0,25,0.5,1,2,4,6, and 24 hours after dosing. In order to obtain three independent plasma concentration time courses, eye bleeds (150 μL) are obtained semilongitudinally using three groups of three animals each, taking two to three time points per animal. Using four volumes of acetonitrile containing an internal standard, plasma samples and controls (25 μL) are extracted, and liquid chromatography tandem mass spectrometry is used for analysis.
Pharmacokinetic studies[1] Female NU/NU or severe combined immunodeficient mice were purchased from Charles River Laboratories or Harlan. AC220 (hydrochloride salt) was formulated in 22% hydroxypropyl-β-cyclodextrin, CEP-701 was formulated in 20% gelucire 44/14 in water (vol/vol), MLN-518 and sunitinib were formulated in 10 mM sodium citrate (pH 3.5), PKC-412 was formulated in 3:1 gelucire 44/14–propylene glycol (vol/vol), and sorafenib (toluene sulfonate salt) was formulated in 80% PEG-400. Compound concentrations were chosen to deliver the desired dose in a volume of 10 mL/kg. Compounds were administered by oral gavage and plasma samples collected 0.25, 0.5, 1, 2, 4, 6, and 24 hours after dosing. To collect plasma samples, eye bleeds (150 μL) were taken semilongitudinally using 3 groups of 3 animals each, taking 2 to 3 time points per animal to obtain a total of 3 independent plasma concentration time courses. Plasma samples and controls (25 μL) were extracted with 4 volumes of acetonitrile containing an internal standard and analyzed by liquid chromatography tandem mass spectrometry. Pharmacokinetic parameters were obtained by fitting the normalized liquid chromatography tandem mass spectrometry peak areas to a noncompartmental model using the linear trapezoidal estimation method in the WinNonlin software package. Mouse studies at Ambit complied with the recommendations of the “Guide for Care and Use of Laboratory Animals”45 with respect to restraint, husbandry, surgical procedures, feed and fluid regulation, and veterinary care. Animal efficacy studies[1] Subcutaneous xenograft model.[1] This model was performed at Ambit to measure in vivo inhibition of FLT3, and by Piedmont Research Center LLC to determine antitumor efficacy, following published procedures. Compounds were formulated and administered as described for pharmacokinetic studies. To measure FLT3 inhibition, tumors were harvested at 2 or 24 hours after compound administration, weighed, and lysed by mechanical dissociation. Tumor lysates were cleared of protein and tissue fragments by centrifugation at 835g for 15 minutes. Cleared lysates were assayed for total and phosphorylated FLT3 using the electrochemiluminescence-based enzyme-linked immunoassay (ELISA) described in “Cellular assays.” Bone marrow engraftment model.[1] The model was performed according to published procedures.20 For intravenous bone marrow engraftment, nonobese diabetic/severe combined immunodeficient mice were acclimated for 2 weeks before pretreatment with 150 mg/kg cyclophosphamide delivered intraperitoneally once a day for 2 days. After a 48-hour rest period, animals were given an intravenous injection of 5 × 106 MV4-11 cells into the tail vein. AC220 was formulated and delivered as described for pharmacokinetic studies. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The mean (SD) absolute bioavailability of quizartinib from the tablet formulation was 71% (±7%) in healthy subjects. After oral administration under fasted conditions, time to peak concentration (median Tmax) of quizartinib and AC886 measured post dose was approximately 4 hours (range 2 to 8 hours) and 5 to 6 hours (range 4 to 120 hours), respectively, in healthy subjects. Following the administration of 35.4 mg quizartinib once daily in patients with newly diagnosed acute myeloid leukemia, the Cmax and AUC0-24h were calculated to be 140 ng/mL (71%) and 2,680 ng.h/mL (85%) respectively during the induction therapy and 204 ng/mL (64%) and 3,930 ng.h/mL (78%) respectively during the consolidation therapy. For the metabolite AC886, the Cmax and AUC0-24h were estimated to be 163 ng/mL (52%) and 3,590 ng.h/mL (51%) respectively during the induction therapy and 172 ng/mL (47%) and 3,800 ng.h/mL (46%) respectively during the consolidation therapy. Increasing the once daily dose of quizartinib to 53 mg also increases the Cmax and AUC0-24h of quizartinib to 529 ng/mL (60%) and 10,200 ng.h/mL (75%) respectively at steady state. The Cmax and AUC0-24h of the metabolite AC886 also increases to 262 ng/mL (48%) and 5,790 ng•h/mL (46%) respectively. No clinically significant differences in the pharmacokinetics of quizartinib were observed when administered with a high-fat, high-calorie meal. Following a single radiolabeled dose of quizartinib 53 mg to healthy subjects, 76.3% of the total radioactivity was recovered in feces (4% unchanged) and 1.64% in urine. Volume of distribution at steady state in healthy subjects was estimated to be 275 L (17%). Total body clearance of quizartinib in healthy subjects was estimated to be 2.23 L/hour (29%). Metabolism / Metabolites In vitro quizartinib is primarily metabolized via oxidation by CYP3A4/5 and AC886 is formed and metabolized by CYP3A4/5. Biological Half-Life The mean (SD) effective half-lives (t1/2) in patients with newly diagnosed AML for quizartinib and AC886 during maintenance therapy are 81 hours (±73) and 136 hours (±113), respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure clinical trials of quizartinib in patients with AML, ALT elevations were arose in 10% to 16% of patients and were above 5 times the upper limit of normal (ULN) in 1% to 3%. However, similar rates were reported in subjects receiving chemotherapy without quizartinib and in most instances the elevations were transient, asymptomatic, and not associated with elevations in serum bilirubin. Intermittent elevations in liver enzymes are not uncommon in patients with untreated AML due to bacterial, viral and opportunistic infections. In the registration trials of quizartinib there were uncommon instances of acute liver injury and hepatic failure, but all were attributable to other comorbidities and factors (multiorgan failure), and none were considered due to quizartinib. Since its approval in the United States, there have been no reported cases of clinically apparent liver injury associated with quizartinib therapy. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of quizartinib during breastfeeding. Because quizartinib is more than 99% bound to plasma proteins, the amount in milk is likely to be low. However, the manufacturer recommends that breastfeeding be discontinued during quizartinib therapy and for 1 month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In vitro plasma protein binding of quizartinib and AC886 is 99% or greater. In vitro blood-to-plasma ratio for quizartinib and AC886 ranges from 0.79-1.30 and 1.36-3.19, respectively. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Quizartinib showed antitumor activity in a mouse model of FLT3-ITD-dependent leukemia. In vitro, studies have shown that quizartinib is a predominant inhibitor of the slow delayed rectifier potassium current, IKs. In AML patients receiving quizartinib at a dose of 90 mg/day for females and 135 mg/day for males on a 28-day schedule, the median levels of phospho-FLT3 (pFLT3) and total FLT3 (tFLT3) decreased from 3312 RLU or 5639 RLU respectively at day 1 to 1235 RLU and 142 RLU respectively at day 8. Additionally, pFLT3 levels are statistically significantly higher (p < 0.0001, Mann Whitney test) for the ITD+ subjects on day 1; however, pFLT3 levels was reduced to a similar level in patients with or without the ITD mutation. The exposure-response analysis predicted a concentration-dependent QTcF interval median prolongation of 18 and 24 ms [upper bound of 2-sided 90% confidence interval (CI): 21 and 27 ms] at the median steady-state Cmax of quizartinib at the 26.5 mg and 53 mg dose level during maintenance therapy. |
| 分子式 |
C29H32N6O4S
|
|---|---|
| 分子量 |
560.67
|
| 精确质量 |
560.22
|
| 元素分析 |
C, 62.13; H, 5.75; N, 14.99; O, 11.41; S, 5.72
|
| CAS号 |
950769-58-1
|
| 相关CAS号 |
1132827-21-4 (HCl);950769-58-1;
|
| PubChem CID |
24889392
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.691
|
| LogP |
4.03
|
| tPSA |
134.4
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
849
|
| 定义原子立体中心数目 |
0
|
| SMILES |
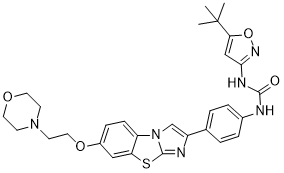
O=C(NC1C=CC(C2=CN3C(SC4C3=CC=C(OCCN3CCOCC3)C=4)=N2)=CC=1)NC1C=C(C(C)(C)C)ON=1
|
| InChi Key |
CVWXJKQAOSCOAB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36)
|
| 化学名 |
1-(5-tert-butyl-1,2-oxazol-3-yl)-3-[4-[6-(2-morpholin-4-ylethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl]urea
|
| 别名 |
Quizartinib; AC220 or AC010220; AC 220; Quizartinib; 950769-58-1; AC220; Quizartinib (AC220); 1-(5-(tert-butyl)isoxazol-3-yl)-3-(4-(7-(2-morpholinoethoxy)benzo[d]imidazo[2,1-b]thiazol-2-yl)phenyl)urea; Quizartinib HCl; AC-220; AC-010220; AC 010220;Vanflyta
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (1.78 mM) (饱和度未知) in 10% DMF 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (1.78 mM) (饱和度未知) in 10% DMF 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 1 mg/mL (1.78 mM) in 10% DMF 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 15% Captisol: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7836 mL | 8.9179 mL | 17.8358 mL | |
| 5 mM | 0.3567 mL | 1.7836 mL | 3.5672 mL | |
| 10 mM | 0.1784 mL | 0.8918 mL | 1.7836 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02668653 | Active Recruiting |
Drug: Quizartinib Drug: Placebo |
Leukemia Acute Myeloid Leukemia |
Daiichi Sankyo, Inc. | September 2016 | Phase 3 |
| NCT04107727 | Active Recruiting |
Drug: Quizartinib Drug: Cytarabine |
Acute Myeloid Leukemia | PETHEMA Foundation | September 5, 2019 | Phase 2 |
| NCT04493138 | Recruiting | Drug: Quizartinib Drug: Azacitidine |
Myelodysplastic Syndrome Myeloproliferative Neoplasm |
M.D. Anderson Cancer Center | July 21, 2020 | Phase 1 Phase 2 |
| NCT04128748 | Recruiting | Drug: Quizartinib Drug: Liposome-encapsulated Daunorubicin-Cytarabine |
Acute Myeloid Leukemia High Risk Myelodysplastic Syndrome |
M.D. Anderson Cancer Center | May 27, 2020 | Phase 1 Phase 2 |
|
 |