| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Opioid receptor
Neutral endopeptidase (NEP, EC 3.4.24.11), Ki=4 nM (human recombinant NEP) [1] IC50=7 nM (rat intestinal tissue NEP) [2] |
|---|---|
| 体外研究 (In Vitro) |
在重组人NEP酶活性实验中,Racecadotril (acetorphan) 的活性代谢产物thiorphan可浓度依赖性抑制NEP活性,Ki=4 nM,在10 nM浓度时抑制率达92%[1]
大鼠肠道组织匀浆实验中,Racecadotril (acetorphan) 对NEP的IC50=7 nM,1 μM浓度时可完全阻断NEP介导的血管活性肠肽(VIP)降解[2] 体外肠上皮细胞单层实验中,100 nM Racecadotril (acetorphan) 可抑制PGE2诱导的氯离子分泌,分泌量较模型组减少58%,且不影响细胞紧密连接完整性[1] |
| 体内研究 (In Vivo) |
在小鼠中,静脉注射给药后,消旋卡多曲迅速代谢为噻吩芬;因此,注射后 30 分钟,从肾脏中仅回收到噻吩芬,而未检测到母体化合物消旋卡多曲 [1]。在大鼠中,单剂量(10 mg/kg)放射性标记的消旋卡多曲 92% 在 24 小时内被消除[1]。 Racecadotril 是一种中性肽链内切酶 (NEP) 抑制剂,已知具有动物抗腹泻活性。口服消旋卡多曲 (100 mg/kg) 在蓖麻油引起的腹泻大鼠模型中显示出疗效[2]。动物模型:6至7周龄雄性Wistar大鼠[2] 剂量:100 mg/kg 给药方法:单次口服治疗;施用蓖麻油前 30 分钟。结果:排便量显着减少,但不能预防腹泻的发生(发生率 100%)。
大鼠蓖麻油诱导急性分泌性腹泻模型中,口服Racecadotril (acetorphan) 剂量依赖性抑制腹泻,10 mg/kg剂量时腹泻发生率降低65%,粪便含水量从89%降至62%,排便次数减少47%[2] 该模型中,30 mg/kg口服剂量的止泻效果与1 mg/kg洛哌丁胺相当,但起效更快(给药后1小时 vs 2小时)[2] 大鼠PGE2诱导腹泻模型中,口服Racecadotril (acetorphan) 5 mg/kg可使粪便含水量减少53%,肠液分泌量降低41%,同时升高肠道组织中VIP和降钙素基因相关肽(CGRP)的浓度(分别增加38%和45%)[1] 小鼠腹泻模型中,口服Racecadotril (acetorphan) 20 mg/kg可缩短腹泻持续时间,从12小时降至5小时,且未观察到便秘副作用[1] |
| 酶活实验 |
重组NEP酶抑制实验:将梯度浓度的Racecadotril (acetorphan) 与重组人NEP及荧光底物共同孵育,37℃温育30分钟后,检测荧光产物的生成量,通过非线性回归分析计算Ki值,评估药物对NEP的抑制亲和力[1]
肠道组织NEP活性检测:制备大鼠肠道组织匀浆,离心获取上清液(含内源性NEP),加入不同浓度的Racecadotril (acetorphan) 和放射性标记的VIP底物,孵育后分离未降解的底物,通过放射性计数定量NEP活性,计算IC50值[2] |
| 细胞实验 |
肠上皮细胞分泌实验:将结肠上皮细胞接种于Transwell小室,培养至形成完整单层(跨上皮电阻值稳定),预先用10-1000 nM Racecadotril (acetorphan) 孵育1小时,再加入PGE2刺激分泌,通过检测跨上皮氯离子电流变化,评估药物对分泌功能的抑制作用[1]
细胞紧密连接完整性检测:上述细胞实验结束后,采用荧光素黄通透性实验检测细胞单层的屏障功能,计算荧光素黄的跨膜渗透率,验证药物是否影响上皮细胞完整性[1] |
| 动物实验 |
6-to-7-week-old male Wistar rats
100 mg/kg A single oral treatment; 30 minutes before castor oil administration. Rat castor oil-induced diarrhea model: Male Wistar rats were acclimated and fasted for 12 hours, then randomly grouped. The experimental group received oral administration of 2.5, 5, 10, 30 mg/kg Racecadotril (acetorphan), with the drug dissolved in normal saline containing 5% polyethylene glycol at an administration volume of 10 mL/kg. The control group received the same volume of vehicle. Thirty minutes after administration, castor oil (10 mL/kg) was intragastrically administered, and the diarrhea incidence, number of defecations, and fecal water content were observed within 4 hours [2] Rat PGE2-induced diarrhea model: Male SD rats were fasted for 12 hours, then the experimental group received oral 5 mg/kg Racecadotril (acetorphan) (dissolved as above). Thirty minutes later, PGE2 (10 μg/kg) was intraperitoneally injected, and diarrhea symptoms were observed within 6 hours. After the experiment, the rats were sacrificed, and the proximal small intestinal tissue was collected to detect VIP and CGRP concentrations [1] Mouse diarrhea model: Female ICR mice were fasted for 8 hours, orally administered 20 mg/kg Racecadotril (acetorphan), and 30 minutes later, senna leaf extract (0.2 g/mL) was intragastrically administered. The onset time, duration of diarrhea, and defecation volume were recorded [1] |
| 药代性质 (ADME/PK) |
After oral administration, Racecadotril (acetorphan) was rapidly absorbed. In humans, after a single oral dose of 100 mg, the time to peak concentration (Tmax)=1.5 hours, and the peak plasma concentration (Cmax)=0.8 μg/mL [1]
The oral bioavailability was approximately 30%. It was rapidly hydrolyzed to the active metabolite thiorphan in the intestinal mucosa and liver, with thiorphan's Tmax=2 hours and Cmax=0.3 μg/mL [1] The elimination half-life (t1/2) of thiorphan=2.5 hours, plasma clearance=15 mL/min/kg, and volume of distribution (Vd)=0.5 L/kg [1] It was mainly excreted through the kidneys, with approximately 65% of metabolites excreted in urine and 15% in feces within 24 hours after administration [1] It could cross the placental barrier, but the fetal plasma concentration was only 12% of that in the mother; a small amount was secreted into breast milk, with the concentration in breast milk about 8% of the maternal plasma concentration [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In the acute toxicity test in rats, the LD50 of oral Racecadotril (acetorphan) was >2000 mg/kg, and the LD50 of intraperitoneal injection was >1000 mg/kg [1]
In the long-term toxicity test in dogs, oral administration of 300 mg/kg daily for 6 months did not cause obvious toxic symptoms, and no abnormalities were found in liver and kidney function, hematological indicators, or histopathological examination [1] Human plasma protein binding rate: thiorphan was approximately 90%, mainly binding to albumin [1] No obvious drug-drug interactions were found. When combined with loperamide, antibiotics, or antihistamines, no enhanced efficacy or toxicity accumulation was observed [1] There were no significant differences in pharmacokinetic parameters between children and the elderly, and no dose adjustment was required [1] |
| 参考文献 |
|
| 其他信息 |
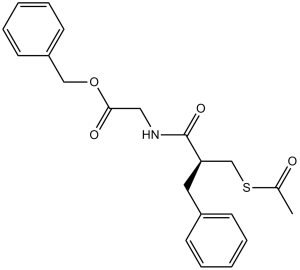
2-[[2-[(acetylthio)methyl]-1-oxo-3-phenylpropyl]amino]acetic acid (phenylmethyl) ester is a N-acyl-amino acid.
Racecadotril has been investigated for the basic science and treatment of Diarrhea, Acute Diarrhea, and Acute Gastroenteritis. Racecadotril (acetorphan) is a prodrug. After oral administration, it is converted to the active metabolite thiorphan by esterases in the intestine and liver. It exerts an antidiarrheal effect by specifically inhibiting NEP, reducing the degradation of antisecretory peptides (such as VIP and CGRP) in the intestine, and regulating intestinal water and sodium balance [1][2] The clinical indication is acute diarrhea, suitable for adults and children (including neonates). It can shorten the duration of diarrhea, reduce the volume and frequency of defecation, without affecting normal intestinal peristalsis and no risk of constipation [1] Compared with loperamide, Racecadotril (acetorphan) has better efficacy in secretory diarrhea, especially suitable for infectious diarrhea (such as rotavirus infection), and does not affect the excretion of intestinal pathogens [1][2] Clinical recommended dose: 100 mg per dose for adults, 3 times a day; 5 mg/kg/day for children, divided into 3 doses, with a course of treatment not exceeding 7 days [1] |
| 分子式 |
C21H23NO4S
|
|
|---|---|---|
| 分子量 |
385.48
|
|
| 精确质量 |
385.134
|
|
| 元素分析 |
C, 65.43; H, 6.01; N, 3.63; O, 16.60; S, 8.32
|
|
| CAS号 |
81110-73-8
|
|
| 相关CAS号 |
Racecadotril-d5; 1246815-11-1
|
|
| PubChem CID |
107751
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
574.5±50.0 °C at 760 mmHg
|
|
| 熔点 |
89ºC
|
|
| 闪点 |
301.2±30.1 °C
|
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
|
| 折射率 |
1.579
|
|
| LogP |
3.44
|
|
| tPSA |
97.77
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
11
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
485
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(CNC(C(CC1C=CC=CC=1)CSC(C)=O)=O)OCC1C=CC=CC=1
|
|
| InChi Key |
ODUOJXZPIYUATO-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H23NO4S/c1-16(23)27-15-19(12-17-8-4-2-5-9-17)21(25)22-13-20(24)26-14-18-10-6-3-7-11-18/h2-11,19H,12-15H2,1H3,(H,22,25)
|
|
| 化学名 |
benzyl 2-[[2-(acetylsulfanylmethyl)-3-phenylpropanoyl]amino]acetate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.49 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.49 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.49 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5942 mL | 12.9708 mL | 25.9417 mL | |
| 5 mM | 0.5188 mL | 2.5942 mL | 5.1883 mL | |
| 10 mM | 0.2594 mL | 1.2971 mL | 2.5942 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05600062 | Recruiting | Drug: Placebo Drug: Racecadotril 100 milligram (MG) Oral Capsule |
Acute Respiratory Distress Syndrome |
Queen Mary University of London |
August 24, 2023 | Not Applicable |
| NCT01577043 | Completed | Drug: Racecadotril Drug: Placebo |
Acute Diarrhea Acute Gastroenteritis |
Centro Pediatrico Albina de Patino |
September 2011 | Phase 4 |
| NCT05216822 | Completed | Drug: Racecadotril | Acute Watery Diarrhea | Assiut University | June 1, 2018 | Phase 1 |
| NCT01153854 | Completed | Drug: Racecadotril Drug: Placebo groups |
Diarrhea | National Institute of Pediatrics, Mexico |
January 2007 | Phase 3 |
| NCT03473561 | Completed | Drug: Racecadotril plus ORS | Diarrhea, Infantile | Abbott | August 25, 2018 | Phase 3 |
|
|
|