| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g | |||
| Other Sizes |

| 靶点 |
Human Liver Aldehyde Oxidase (AO): Raloxifene HCl inhibits human liver AO activity with a Ki value of 1.2 μM, showing competitive inhibition against AO substrate phthalazine [2]
- Estrogen Receptor α/β (ERα/β): Raloxifene HCl binds to ERα and ERβ as a selective modulator,It exhibits agonist activity in bone tissue and antagonist activity in mammary/uterine tissue [1][3][4] |
|---|---|
| 体外研究 (In Vitro) |
在纳摩尔浓度下,雷洛昔芬完全激动地激活 TGF beta 3 启动子。在瞬时转染研究中,雷洛昔芬作为纯雌激素拮抗剂,抑制卵黄蛋白原启动子的表达,该启动子含有雌激素反应元件[1]。雷洛昔芬的 Ki 值范围为 0.87 至 1.4 nM,是人肝醛氧化酶氧化二氮杂萘、香草醛和尼古丁的强非竞争性抑制剂[2]。雷洛昔芬的 Ki 值为 51 nM,也是醛氧化酶催化的含异羟肟酸分子还原过程的非竞争性抑制剂[2]。 Raloxifene(0-80 μM;48 小时)以浓度依赖性方式显着降低小鼠乳腺癌 BJMC3879luc2 细胞的活力[5]。
1. 抑制人肝脏醛脱氢酶([2]): - 人肝脏胞质AO(0.5 mg/mL)与盐酸雷洛昔芬(0.1–10 μM)及AO底物酞嗪(100 μM)在37°C孵育60分钟,以浓度依赖方式抑制AO介导的酞嗪氧化。1 μM浓度下抑制率为45%,10 μM浓度下抑制率达90%(HPLC检测酞嗪代谢产物定量)[2] 2. 对转移性乳腺癌细胞的抗增殖活性([5]): - 用盐酸雷洛昔芬(1–50 μM)处理MDA-MB-231(ER阴性,转移性乳腺癌)细胞72小时,抑制细胞增殖,MTT实验显示IC50为8 μM。20 μM浓度下,诱导凋亡率35%(Annexin V/PI流式细胞术),降低迁移能力40%(Transwell迁移实验)[5] |
| 体内研究 (In Vivo) |
在卵巢切除 (OVX) 大鼠中,雷洛昔芬(3 mg/kg;每日一次)对子宫湿重的影响有限,并且对骨吸收和血清胆固醇的雌激素活性较低[3]。在近端胫骨和远端股骨中,雷洛昔芬(口服剂量;0.1 mg/kg-10 mg/kg;5周)可增强骨矿物质密度。在卵巢切除 (OVX) 大鼠中,它可降低血清胆固醇,ED50 为 0.2 mg/kg[4]。雷洛昔芬(皮下植入微型渗透泵;18 或 27 mg/kg;每天一次;6 周)除了显着抑制小鼠肿瘤大小外,还显着减少淋巴结转移的数量[5]。
1. 刺激大鼠骨组织中TGF-β3表达([1]): - 去卵巢(OVX)雌性SD大鼠(250–300 g)口服给予1 mg/kg/天盐酸雷洛昔芬,连续14天。股骨组织分析显示,与OVX对照组相比,盐酸雷洛昔芬使TGF-β3 mRNA表达上调60%(实时PCR),蛋白水平上调55%(蛋白质印迹法),同时使骨小梁密度增加30%(micro-CT检测)[1] 2. 对去卵巢大鼠生殖/非生殖组织的影响([3]): - OVX大鼠口服给予盐酸雷洛昔芬(0.1、1、10 mg/kg/天),连续21天。与雌激素(1 μg/kg/天)或他莫昔芬(1 mg/kg/天)不同,盐酸雷洛昔芬不增加子宫湿重(子宫重量/体重比与OVX对照组无差异)。10 mg/kg剂量下,使胫骨骨密度(BMD)增加25%,血清低密度脂蛋白(LDL)胆固醇降低20% [3] 3. 预防骨丢失与降低胆固醇([4]): - OVX大鼠口服给予盐酸雷洛昔芬(1、5、10 mg/kg/天),连续8周。10 mg/kg剂量抑制股骨骨丢失40%(BMD检测),降低血清总胆固醇25%、甘油三酯15%(酶法检测),且未引起子宫肥大(子宫重量与OVX对照组相近)[4] 4. 抑制肿瘤生长与转移([5]): - 6–8周龄雌性裸鼠皮下接种2×10⁶ MDA-MB-231细胞,当肿瘤体积达100 mm³时,口服给予盐酸雷洛昔芬(5、10、20 mg/kg/天),连续28天。20 mg/kg剂量使肿瘤体积缩小50%、肿瘤重量降低45%(每周两次测量),同时使腋窝淋巴结转移率从对照组的80%降至30%(组织学检查)[5] |
| 酶活实验 |
人肝脏醛脱氢酶(AO)抑制实验([2]):
反应体系为200 μL,含50 mM磷酸钾缓冲液(pH 7.4)、0.5 mg/mL人肝脏胞质(AO来源)、100 μM酞嗪(AO底物)及盐酸雷洛昔芬(0.1–10 μM)。37°C孵育60分钟后,加入50 μL 10%三氯乙酸终止反应。离心(10,000×g,10分钟)后,上清液经HPLC(C18柱)分析,定量未代谢的酞嗪。通过Lineweaver-Burk双倒数作图分析竞争性抑制,计算Ki值 [2] |
| 细胞实验 |
细胞活力测定[5]
细胞类型: BJMC3879luc2 细胞 测试浓度: 0 μM、10 μM、20 μM、40 μM、80 μM 孵育持续时间:48 小时 实验结果:BJMC3879luc2 细胞活力降低。 MDA-MB-231细胞增殖、凋亡与迁移实验([5]): 1. 增殖实验:96孔板每孔接种5×10³ MDA-MB-231细胞,用含10%胎牛血清的RPMI 1640培养24小时后,加入盐酸雷洛昔芬(1–50 μM),孵育72小时。加入MTT试剂,570 nm处测吸光度计算IC50。 2. 凋亡实验:6孔板每孔接种2×10⁵细胞,用20 μM 盐酸雷洛昔芬处理48小时。Annexin V-FITC/PI染色后,流式细胞术计数凋亡细胞。 3. 迁移实验:Transwell小室(8 μm孔径)包被胶原,上室加入1×10⁴细胞(无血清培养基+20 μM 盐酸雷洛昔芬),下室加入含10%胎牛血清的培养基。24小时后,结晶紫染色下室膜上迁移细胞并计数 [5] |
| 动物实验 |
Animal/Disease Models: Syngeneic balb/c (Bagg ALBino) mouse with BJMC3879luc2 cells[5]
Doses: 18 or 27 mg/kg Route of Administration: subcutaneously (sc) implanted mini-osmotic pumps Experimental Results: Inhibited tumor growth in mice. 1. OVX Rat Bone TGF-β3 Expression Protocol ([1]): - Ovariectomy: Female Sprague-Dawley rats (250–300 g) were anesthetized and subjected to bilateral ovariectomy; sham-operated rats served as control. - Drug Preparation: Raloxifene HCl was dissolved in 0.5% methylcellulose + 0.1% Tween 80. - Administration: OVX rats were orally gavaged with 1 mg/kg/day Raloxifene HCl or vehicle for 14 days; sham rats received vehicle. - Sample Collection: Rats were euthanized, femurs were collected for micro-CT (BMD measurement) and RNA/protein extraction (TGF-β3 detection) [1] 2. OVX Rat Tissue Effect Protocol ([3]): - Ovariectomy: As described in [1]; OVX rats were randomized into 4 groups (n=6/group). - Administration: Rats were orally gavaged with Raloxifene HCl (0.1, 1, 10 mg/kg/day), estrogen (1 μg/kg/day), or vehicle for 21 days. - Tissue Analysis: Uterine wet weight was measured; tibial BMD was detected via dual-energy X-ray absorptiometry (DXA); serum LDL cholesterol was quantified via enzymatic assay [3] 3. OVX Rat Bone Loss/Cholesterol Protocol ([4]): - Ovariectomy: As described in [1]; OVX rats were randomized into 4 groups (n=8/group). - Administration: Rats were orally gavaged with Raloxifene HCl (1, 5, 10 mg/kg/day) or vehicle for 8 weeks. - Detection: Femoral BMD was measured via DXA; serum total cholesterol and triglycerides were analyzed via commercial kits [4] 4. MDA-MB-231 Xenograft Protocol ([5]): - Cell Inoculation: 2×10⁶ MDA-MB-231 cells (suspended in 0.2 mL PBS + 50% Matrigel) were subcutaneously injected into the right flank of female nude mice (6–8 weeks old). - Administration: When tumors reached 100 mm³, mice were orally gavaged with Raloxifene HCl (5, 10, 20 mg/kg/day) or vehicle for 28 days. - Tumor/Metastasis Detection: Tumor volume was calculated as (length × width²)/2 twice weekly. After euthanasia, tumors were weighed; axillary lymph nodes were sectioned and stained with H&E to assess metastasis [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Raloxifene is well absorbed from the gastrointestinal tract, with approximately 60% fo the drug being absorbed following oral administration. Due to the extensive first-pass hepatic metabolism that involves glucuronide conjugation, the absolute oral bioavailability of raloxifene is about 2%. Following oral ingestion of a single dose or multiple dose of raloxifen in healthy postmenopausal women, the mean peak plasma concentrations (Cmax) were 0.50 and 1.36 ng/mL, respectively, and the AUC values were 27.2 and 24.2 ngxhr/mL, respectively. The time to reach Cmax following a single or multiple oral doses were 27.7 and 32.5 hours, respectively. Although not clinically significant, oral ingestion of raloxifene with high-fat meals is thought to increase the systemic bioavailability of the drug by increasnig the peak plasma concentrations (Cmax) and AUC by 28% and 16%, respectively. Raloxifene predominantly undergoes fecal excretion, with less than 0.2% of the dose being excreted in the urine as unchanged form of the compound and less than 6% of the dose being excreted as glucuronide conjugates. Co-administration with [cholestyramine], a bile acid sequestrant, was shown to reduce the enterohepatic recycling of raloxifene by 60%. Following oral administration of single doses randing from 30 to 150 mg in postmenopausal women, the volume of distribution was about 2348 L/kg. Following oral administration of multiple doses, the value increased to 2853 L/kg. Raloxifene is widely distributed in the tissues. It is not known whether raloxifene is excreted in human milk. Following intravenous administration, raloxifene was shown to be cleared at a rate approximating hepatic blood flow. The apparent oral clearance is reported to be 44.1 L/kgxhr. The clearance can range from 40 to 60L/kgxhr following chronic dosing. In healthy postmenopausal women receiving multiple oral dose, the mean clearance was 47.4 L/kgxhr. Apparent clearance can be reduced by 56% in patients with hepatic impairment. It is not known whether raloxifene crosses the human placenta. The molecular weight (about 474 for the free base) and the long elimination half life suggest that the drug will cross to the embryo-fetus. However, the high plasma protein binding might limit the exposure. Raloxifene undergoes extensive first-pass glucuronidation and enterohepatic circulation, and peak plasma concentrations of the glucuronide conjugates of raloxifene are achieved more rapidly than peak plasma concentrations of the parent drug. Following oral administration of a single 120- or 150-mg dose of raloxifene hydrochloride, peak plasma concentrations of raloxifene and its glucuronide conjugates are achieved at 6 and 1 hour, respectively. Plasma concentrations of raloxifene's glucuronide conjugates exceed those of the parent drug, and the time to achieve maximum concentrations of the drug and glucuronide metabolites depends on the extent and rate of systemic interconversion and enterohepatic circulation. Following oral administration of radiolabeled raloxifene, less than 1% of total circulating radiolabeled material in plasma represented parent drug. The apparent volume of distribution following oral administration of single doses of raloxifene hydrochloride 30-150 mg is 2348 L/kg, suggesting extensive tissue distribution. The volume of distribution reportedly is not dose dependent over a dosage range of 30-150 mg daily. Raloxifene is excreted principally in feces as unabsorbed drug and via biliary elimination as glucuronide conjugates, which subsequently are metabolized by bacteria in the GI tract to the parent drug. Following oral administration, less than 6 or 0.2% of a raloxifene dose is excreted as glucuronide conjugates or parent drug, respectively, in urine. For more Absorption, Distribution and Excretion (Complete) data for Raloxifene (11 total), please visit the HSDB record page. Metabolism / Metabolites Raloxifene is reported to undergo metabolism in the intestines and liver devoid of cytochrome P450 pathway. It is extensively metabolized, where less than 1% of the total dose exists as unchanged compound. It mainly undergoes first-pass metabolism to form glucuronide conjugates, raloxifene-4'-glucuronide (raloxifene-4'-β-glucuronide), raloxifene-6-glucuronide (raloxifene-6-β-glucuronide), and raloxifene-6,4'-diglucuronide. No other metabolites have been detected in human plasma. The terminal log-linear portions of the plasma concentration curves for raloxifene and the glucuronides are generally parallel. This is consistent with interconversion of raloxifene and the glucuronide metabolites. Biotransformation and disposition of raloxifene in humans have been determined following oral administration of (14)C-labeled raloxifene. Raloxifene undergoes extensive first-pass metabolism to the glucuronide conjugates: raloxifene-4'-glucuronide, raloxifene-6-glucuronide, and raloxifene-6, 4'-diglucuronide. No other metabolites have been detected, providing strong evidence that raloxifene is not metabolized by cytochrome P450 pathways. Unconjugated raloxifene comprises less than 1% of the total radiolabeled material in plasma. The terminal log-linear portions of the plasma concentration curves for raloxifene and the glucuronides are generally parallel. This is consistent with interconversion of raloxifene and the glucuronide metabolites. Raloxifene undergoes extensive first-pass metabolism to the glucuronide conjugates raloxifene 4'-glucuronide, 6-glucuronide, and 6,4'-diglucuronide. Metabolism of raloxifene does not appear to be mediated by cytochrome P-450 enzymes, since metabolites other than glucuronide conjugates have not been identified. Raloxifene has known human metabolites that include [6,7-Dihydroxy-2-(4-hydroxyphenyl)benzo[b]thiophene-3-yl][4-(2-piperidinoethoxy)phenyl]methanone, Raloxifene 6-O-glucuronide, and [2-(3,4-dihydroxyphenyl)-6-hydroxy-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone. Biological Half-Life The average plasma elimination half-life of raloxifene ranges from 27 to 32 hours. The prolonged half-life has been attributed to the drug's reversible systemic metabolism and significant enterohepatic cycling. The plasma elimination half-life of raloxifene at steady-state averages 32.5 hours (range: 15.8-86.6 hours). Raloxifene and its glucuronide conjugates are interconverted by reversible systemic metabolism and enterohepatic cycling, thereby prolonging its plasma elimination half-life to 27.7 hours after oral dosing. Oral Absorption: Raloxifene HCl has oral bioavailability of ~2% in humans due to extensive first-pass metabolism (glucuronidation) in the liver and intestine [4] - Plasma Half-Life: In OVX rats, oral administration of 10 mg/kg Raloxifene HCl showed a plasma half-life of 6 hours, with peak plasma concentration (Cmax) of 80 ng/mL at 2 hours post-gavage [4] - Metabolism: Raloxifene HCl is primarily metabolized by UDP-glucuronosyltransferases (UGTs) in the liver to inactive glucuronide conjugates, with no significant metabolism by cytochrome P450 enzymes [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Raloxifene is used for the treatment and prevention of osteoporosis in postmenopausal women and for the reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis. HUMAN STUDIES: No fatalities associated with raloxifene overdose have been reported. In postmarketing reports, adverse reactions were reported in approximately half of the adults who took >/= 180 mg raloxifene HCl and included leg cramps and dizziness. Raloxifene therapy is associated with an increased risk of venous thromboembolic events such as deep-vein thrombosis and pulmonary embolism. Increased risk of death due to stroke occurred in a trial in postmenopausal women with documented coronary heart disease or at increased risk for major coronary events. A few cases of jaw bone osteonecrosis have been associated with raloxifene. Two 18-month-old children each ingested raloxifene HCl 180 mg, symptoms reported included ataxia, dizziness, vomiting, rash, diarrhea, tremor, and flushing, as well as elevation in alkaline phosphatase. Raloxifene may cause fetal toxicity when administered to pregnant women. Effects on reproductive function are expected because raloxifene is an estrogen agonist-antagonist. ANIMAL STUDIES: Mortality was not observed in rats or mice following single oral doses of raloxifene hydrochloride 5000 mg/kg, or in monkeys following single oral doses of raloxifene hydrochloride 1000 mg/kg. In a 21-month carcinogenicity study in mice, there was an increased incidence of ovarian tumors in female mice given oral raloxifene hydrochloride 9-242 mg/kg daily and an increased incidence of testicular interstitial cell tumors, prostatic adenomas, and adenocarcinomas in male mice given raloxifene hydrochloride 41 or 210 mg/kg daily. In studies in rats using raloxifene hydrochloride, doses of 0.1-10 mg/kg during gestation and lactation delayed and disrupted parturition, decreased neonatal survival and altered physical development, sex- and age-specific reductions in growth and changes in pituitary hormone content, and decreased lymphoid compartment size in offspring were observed. Disruption of parturition, which resulted in maternal and progeny morbidity and/or death, was observed in rats given raloxifene hydrochloride 10 mg/kg. While ovarian or vaginal pathology was not observed in adult offspring (4 months of age), uterine hypoplasia and reduced fertility were noted. In reproductive studies in rabbits using raloxifene hydrochloride, doses of 0.1 mg/kg or more resulted in abortion and a low rate of fetal heart anomalies (i.e., ventricular septal defects). In rabbits using raloxifene doses of 10 mg/kg or more (at least 4 times the recommended dose in humans on a mg/sq m basis), hydrocephaly was observed in the fetuses. In female rats, at doses of 0.1 to 10 mg/kg/day, raloxifene disrupted estrous cycles and inhibited ovulation. These effects of raloxifene were reversible. When male and female rats were given daily doses >/=5 mg/kg prior to and during mating, no pregnancies occurred. In reproductive studies in rats using raloxifene hydrochloride doses of 1 mg/kg or more, retardation of fetal development and developmental abnormalities (i.e., wavy ribs, kidney cavitation) were observed. Raloxifene was not mutagenic in in vitro or in vivo studies, including the Ames microbial test with and without metabolic activation, the unscheduled DNA synthesis assay in rat hepatocytes, the mouse lymphoma assay for mammalian cell mutation, the chromosomal aberration assay in Chinese hamster ovary cells, the sister chromatid exchange assay in Chinese hamsters, and the micronucleus test in mice. Protein Binding About 95% of raloxifene and its glucuronide metabolites are bound to plasma proteins. Although this is a relatively high protein binding profile, _in vitro_ studies suggest that raloxifene and its metabolites do not significantly interact with binding of highly protein-bound drugs. FDA Label still advises patients to use raloxifene with caution co-administering with other highly protein-bound drugs. Interactions The manufacturer states that concomitant use of systemic estrogens with raloxifene currently is not recommended because of the lack of experience from prospective clinical trials with such use. Concomitant administration of raloxifene and ampicillin results in a 28% decrease in peak plasma concentration and a 14% decrease in the extent of absorption of raloxifene. These changes in raloxifene absorption are consistent with decreased enterohepatic cycling associated with a reduction of enteric bacteria. Because systemic exposure and the elimination rate of raloxifene are not affected, raloxifene may be given concomitantly with ampicillin. In raloxifene-treated women with osteoporosis, concomitant administration of amoxicillin did not affect the plasma concentrations of raloxifene. Raloxifene may be given concomitantly with amoxicillin. While the effect of long-term administration of raloxifene in conjunction with warfarin has not been studied and the drug reportedly does not affect the protein binding of the anticoagulant, concomitant administration of single doses of raloxifene and warfarin has resulted in a 10% decrease in prothrombin time compared with administration of warfarin alone. In raloxifene-treated women with osteoporosis, concomitant administration of warfarin did not affect the plasma concentrations of raloxifene. If the drugs are used concomitantly, the patient and prothrombin time should be monitored closely and the dosage of the anticoagulant adjusted accordingly. Administration of cholestyramine and raloxifene results in a 60% decrease in the absorption and enterohepatic cycling of raloxifene. The manufacturer states that raloxifene should not be administered with cholestyramine. Although not studied specifically, other anion-exchange resins would also be expected to decrease the absorption and enterohepatic cycling of raloxifene. Raloxifene is more than 95% bound to plasma proteins. The manufacturer states that concomitant administration of raloxifene with other highly protein-bound drugs is not expected to affect the plasma concentrations of raloxifene. In raloxifene-treated women with osteoporosis, concomitant administration of other highly protein-bound drugs (eg, gemfibrozil) did not affect the plasma concentrations of raloxifene. Raloxifene reportedly does not affect the protein binding of phenytoin, tamoxifen, or warfarin in vitro. The manufacturer states that caution is advised if raloxifene is used concomitantly with other highly protein-bound drugs such as diazepam, diazoxide, or lidocaine. 1. In Vitro Cytotoxicity: - Raloxifene HCl (1–50 μM) showed no cytotoxicity in normal human osteoblasts (cell viability >90% vs. control, MTT assay) [5] - At concentrations >100 μM, it induced non-specific cell death in MDA-MB-231 cells (viability <60% vs. control) [5] 2. In Vivo Toxicity: - OVX rats treated with Raloxifene HCl (0.1–10 mg/kg/day) for 8 weeks showed no significant changes in liver function (ALT, AST) or kidney function (BUN, creatinine) compared to control [3][4] - No uterine hypertrophy (a common side effect of estrogen/tamoxifen) was observed in Raloxifene HCl-treated OVX rats [3][4] - Nude mice treated with 20 mg/kg/day Raloxifene HCl for 28 days showed no weight loss or hematological abnormalities (leukocyte, platelet counts normal) [5] 3. Plasma Protein Binding: Raloxifene HCl has high plasma protein binding (>98%) in human and rat plasma (measured via ultrafiltration) [4] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Estrogen Antagonists; Selective Estrogen Receptor Modulators; Bone Density Conservation Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Raloxifene is included in the database. Evista is indicated for the treatment and prevention of osteoporosis in postmenopausal women. /Included in US product label/ Evista is indicated for the reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis. /Included in US product label/ For more Therapeutic Uses (Complete) data for Raloxifene (14 total), please visit the HSDB record page. Drug Warnings Use of tamoxifen has been associated with increased rates of cataracts and cataract surgery. In the STAR study, fewer cataracts (RR 0.79; 95% confidence interval: 0.68-0.92) and cataract surgeries (RR 0.82; 95% confidence interval: 0.68-0.99) occurred in those receiving raloxifene than in those receiving tamoxifen. Use of raloxifene did not affect the risk of coronary events in a study in postmenopausal women with coronary heart disease (CHD) or risk factors for CHD (RUTH study). In the STAR study, the incidence of ischemic heart disease (i.e., myocardial infarction, severe angina, acute ischemic syndrome) in those receiving raloxifene was similar to the incidence in those receiving tamoxifen. The percentage of sexually active women was lower with raloxifene compared with tamoxifen at nearly every assessment point over the 5-year study duration; among sexually active women, there were increased reports of difficulty with sexual arousal, interest and enjoyment in women receiving raloxifene. Syncope or development of a varicose vein condition1 has occurred in up to 2.3% of patients receiving raloxifene in clinical studies. In clinical trials, peripheral edema occurred in up to 14.1% of raloxifene-treated women. For more Drug Warnings (Complete) data for Raloxifene (25 total), please visit the HSDB record page. Pharmacodynamics Raloxifene belongs to the selective estrogen receptor modulator (SERM) drug class that exhibits estrogenic effects on bone and lipid metabolism while mediating anti-estrogenic effects on uterine endometrium and breast tissues. On skeletal tissues, raloxifene stimulates bone-depositing osteoblasts and inhibits bone-resorbing osteoclasts to augument bone mineral density. Raloxifene produces estrogen-like effects on bone, reducing the resorption of bone and increasing bone mineral density in postmenopausal women, thus slowing the rate of bone loss. In three randomized, placebo-controlled trials in Europe, postmenopausal women receiving raloxifene at variable doses of 30 to 150 mg daily demonstrated significant increases in bone mineral density in the lumbar spine, total hip, femoral neck and total body compared to placebo. In the MORE and RUTH trials, there were fewer incidences of vertebral fractures in postmeopausal women receiving raloxifene compared to placebo. In a eight-week study evaluating short-term effects of raloxifene in healthy postmenopausal women, there was a decrease in the bone turnover markers, such as serum alkaline phosphatase level, serum osteocalcin level and urinary calcium excretion. Raloxifene was shown to inhibit estrogen-dependent proliferation of human breast cancer cells _in vitro_ and development of induced mammary tumors in rats _in vivo_. In adult female rats, raloxifene produced a greater regression of the mammary gland than [tamoxifen]. The MORE trial was a multicenter, randomized, double-blind clinical trial that investigated the long-term effects of the drug therapy in European and American postmenopausal women receiving raloxifene for 40 months. Additionally, a reduction in the incidence of invasive breast cancer was also demonstrates in the CORE and RUTH trials. Study findings demonstrated that compared to placebo, the risk of invasive breast cancer was decreased by 76% among postmenopausal women with osteoporosis. There was a decrease in the risk of estrogen receptor-positive breast cancer by 90% but there was no increase in the risk of endometrial cancer. Unlike hormone replacement therapy, raloxifene does not mediate proliferative or stimulatory effects on endometrial tissue. Findings from both animal and human studies demonstrated no significant changes in the histologic appearance of the endometrium. Raloxifene promotes estrogen-like effects on lipid metabolism. In a European trial that evaluated lipid profiles following raloxifene therapy over the 24-month period, there were significant decreases in the serum concentrations of total and low-density lipoprotein (LDL) cholesterol over a 24-month period of raloxifene therapy. Raloxifene is not associated with causing alterations in the serum levels of HDL cholesterol or triglycerides. As the HDL choesterol level is considered a strong inverse predictor of cardiovascular disease in women, the cardioprotective effects of raloxifene were questioned. Due to limited data on the long-term trials, it is not possible to determine whether the small lipid effects produced by raloxifene correlate with a smaller degree of cardioprotective activity compared with hormone replacement therapy. 1. Drug Classification & Mechanism ([1][3][4]): - Raloxifene HCl is a selective estrogen receptor modulator (SERM) that acts as an ER agonist in bone (stimulates TGF-β3 expression, maintains BMD) and lipid metabolism (reduces LDL cholesterol) and as an ER antagonist in mammary/uterine tissue (prevents cell proliferation) [1][3][4] 2. Indications ([4][5]): - Approved for the prevention and treatment of postmenopausal osteoporosis (via anti-bone loss effects). It also shows potential for inhibiting metastatic breast cancer (reduces tumor growth and lymph node metastasis) [4][5] 3. Advantage Over Other SERMs ([3][4]): - Unlike tamoxifen or estrogen, Raloxifene HCl does not induce uterine hypertrophy, reducing the risk of endometrial cancer associated with long-term estrogen/tamoxifen use [3][4] 4. Role of TGF-β3 in Bone Maintenance ([1]): - Raloxifene HCl-mediated bone protection is partially via upregulating TGF-β3, which promotes osteoblast differentiation and inhibits osteoclast activity, thereby increasing trabecular bone density [1] |
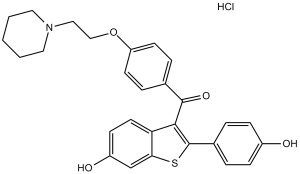
| 分子式 |
C28H27NO4S.HCL
|
|---|---|
| 分子量 |
510.04
|
| 精确质量 |
509.142
|
| CAS号 |
82640-04-8
|
| 相关CAS号 |
Raloxifene;84449-90-1
|
| PubChem CID |
5035
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.285g/cm3
|
| 沸点 |
728.2ºC at 760 mmHg
|
| 熔点 |
143-147ºC
|
| 闪点 |
394.2ºC
|
| 折射率 |
1.654
|
| LogP |
6.815
|
| tPSA |
98.24
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
655
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
GZUITABIAKMVPG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2
|
| 化学名 |
[6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone
|
| 别名 |
LY-156758; LY-139481; LY 156758; LY139481; LY156758 (Keoxifene) HCl; LY 139481; trade names: Evista; Keoxifene; RALOX
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% methylcellulose: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9606 mL | 9.8032 mL | 19.6063 mL | |
| 5 mM | 0.3921 mL | 1.9606 mL | 3.9213 mL | |
| 10 mM | 0.1961 mL | 0.9803 mL | 1.9606 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
STUDY OF CIRCULATING OSTEOBLAST-LINEAGE CELLS IN RELATION WITH TERIPARATIDE THERAPY.
CTID: null
Phase: Phase 4 Status: Ongoing
Date: 2006-08-02