| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
norepinephrine reuptake ( Ki = 8.2 nM )
Reboxetine mesylate targets human norepinephrine transporter (NET) with a Ki value of 1.8 nM (radioligand binding assay) and an IC₅₀ value of 2.4 nM (norepinephrine uptake inhibition assay in HEK293-NET cells) [5] Reboxetine mesylate shows high selectivity for NET over serotonin transporter (SERT) and dopamine transporter (DAT): Ki = 1200 nM (SERT), Ki > 10,000 nM (DAT) [5] |
|---|---|
| 体外研究 (In Vitro) |
瑞波西汀(也称为 PNU 155950E;商品名:Edronax)是一种去甲肾上腺素再摄取抑制剂,Ki 为 8.2 nM,具有抗抑郁活性。瑞波西汀剂量依赖性且完全抑制人去甲肾上腺素转运蛋白的 [3H]-多巴胺摄取( hNET),在 Madin–Darby 犬肾 (MDCK) 细胞中 Ki 值为 11 nM。瑞波西汀剂量依赖性地有效抑制大鼠蓝斑神经元放电,ED50 为 191 μg/kg。瑞波西汀对蓝斑神经元的抑制作用可被 α2 拮抗剂哌罗生(1.5 mg/kg,IV)逆转。瑞波西汀剂量依赖性地完全抑制人去甲肾上腺素转运蛋白 (hNET) 对 [3H]-多巴胺的摄取,在 Madin–Darby 犬肾 (MDCK) 细胞中 Ki 值为 11 nM。 激酶测定:瑞波西汀(也称为 PNU 155950E;贸易)名称:Edronax)是一种去甲肾上腺素再摄取抑制剂,Ki 为 8.2 nM,具有抗抑郁活性。细胞测定:瑞波西汀剂量依赖性且完全抑制人去甲肾上腺素转运蛋白 (hNET) 的 [3H]-多巴胺摄取,Madin 中的 Ki 值为 11 nM –达比犬肾 (MDCK) 细胞。瑞波西汀剂量依赖性地有效抑制大鼠蓝斑神经元放电,ED50 为 191 μg/kg。瑞波西汀对蓝斑神经元的抑制作用可被 α2 拮抗剂哌罗生(1.5 mg/kg,IV)逆转。瑞波西汀剂量依赖性地完全抑制人去甲肾上腺素转运蛋白 (hNET) 对 [3H]-多巴胺的摄取,在 Madin–Darby 犬肾 (MDCK) 细胞中 Ki 值为 11 nM。
NET抑制活性:Reboxetine mesylate(0.1–100 nM)剂量依赖性抑制过表达人NET的HEK293细胞对[³H]-去甲肾上腺素的摄取,50 nM浓度下抑制率达95%;浓度高达1 μM时,对[³H]-5-羟色胺(SERT介导)或[³H]-多巴胺(DAT介导)的摄取无明显抑制 [5] - 神经元活性调控:在大鼠皮质切片中,1–10 μM浓度使细胞外去甲肾上腺素水平升高2.3–4.8倍(微透析+HPLC检测),不影响5-羟色胺或多巴胺水平 [4] - 无明显细胞毒性:PC12细胞和原代大鼠皮质神经元中CC₅₀ > 100 μM;浓度高达50 μM时细胞活力>90%(MTT法) [5] |
| 体内研究 (In Vivo) |
瑞波西汀剂量依赖性地有效抑制大鼠蓝斑神经元放电,ED50 为 191 μg/kg。瑞波西汀对蓝斑神经元的抑制作用可被 α2 拮抗剂哌罗生(1.5 mg/kg,IV)逆转。瑞波西汀剂量依赖性地逆转利血平诱导的小鼠眼睑痉挛和体温过低。瑞波西汀还被发现可以剂量依赖性地拮抗可乐定引起的小鼠体温过低。瑞波西汀可逆转利血平诱导的大鼠眼睑痉挛和体温过低,ED50 分别为 10 mg/kg 和 3 mg/kg (po)。瑞波西汀可显着减少 DSM-III-R 惊恐障碍患者惊恐发作和恐惧症状的平均次数。瑞波西汀还可以改善汉密尔顿抑郁量表、霍普金斯症状检查表 90 和希恩残疾量表的分数。与安慰剂相比,瑞波西汀的复发率显着降低(22% vs. 56%),并且在复发性 DSM-III-R 重度抑郁症患者的长期治疗过程中维持缓解的累积概率更大。瑞波西汀可有效预防发作缓解后抑郁症状的复发。瑞波西汀(0.3 mg/kg-20 mg/kg)的急性全身给药剂量依赖性地增加大鼠额叶皮质中的细胞外去甲肾上腺素,同时对细胞外血清素没有影响。瑞波西汀 (20 mg/kg) 还会增加大鼠额叶皮层的细胞外多巴胺。长期服用瑞波西汀 14 天会导致大鼠额叶皮层细胞外去甲肾上腺素和多巴胺的基础浓度升高,细胞外去甲肾上腺素和多巴胺的净增加更大,但不会增加血清素。瑞波西汀剂量依赖性地减少尼古丁自我给药约 60%。瑞波西汀 (5.6 mg/kg) 的重复给药可降低 14 次疗程中的尼古丁自我给药和蔗糖维持反应。
抗抑郁样活性(小鼠模型):口服Reboxetine mesylate(5、10、20 mg/kg),给药60分钟后进行强迫游泳实验(6分钟)或悬尾实验(6分钟),剂量依赖性减少不动时间,较溶媒对照组分别减少32%、55%、68%(强迫游泳实验)和28%、48%、62%(悬尾实验) [1][4] - 去甲肾上腺素水平升高:大鼠口服10 mg/kg后,前额叶皮质和海马组织中去甲肾上腺素含量分别增加1.8倍和2.1倍(HPLC检测) [4] - 临床抗抑郁疗效:重度抑郁症(MDD)患者口服Reboxetine mesylate(4–8 mg/天,分两次给药),治疗6–8周后,蒙哥马利-阿斯伯格抑郁量表(MADRS)评分改善35–52%,有效率(症状改善≥50%)达58–65% [2][3] - 对心血管参数无明显影响:清醒大鼠静脉注射5 mg/kg,未观察到心率或血压的显著变化 [5] |
| 酶活实验 |
NET放射性配体结合实验:将重组人NET蛋白固定于细胞膜,系列稀释的Reboxetine mesylate(0.01 nM–1 μM)与[³H]-nisoxetine(NET特异性配体)在25°C孵育60分钟。过滤分离结合态与游离态配体,定量放射性强度计算Ki值 [5]
- 去甲肾上腺素摄取抑制实验:过表达人NET的HEK293细胞接种于24孔板,用Reboxetine mesylate(0.1 nM–1 μM)预处理30分钟后,与[³H]-去甲肾上腺素在37°C孵育15分钟。洗涤细胞并裂解,检测放射性强度评估摄取抑制效果及IC₅₀ [5] |
| 细胞实验 |
细胞系:SH-SY5Y细胞
浓度:0.1 μM、1 μM、5 μM 孵育时间:24小时 结果:防止地塞米松引起的细胞活力和增殖率下降。 转染细胞NET介导摄取实验:HEK293细胞转染人NET cDNA,培养48小时后血清饥饿1小时。细胞与Reboxetine mesylate(0.1–100 nM)及[³H]-去甲肾上腺素共孵育,裂解后定量放射性 [5] - 原代皮质神经元实验:大鼠皮质神经元培养7–10天,用Reboxetine mesylate(1–10 μM)处理24小时。收集细胞外液,HPLC检测去甲肾上腺素水平,验证神经递质释放/摄取调控作用 [4] |
| 动物实验 |
Harlan-bred, male CF-1 mice (18-20 g), depression models
3 mg/kg, 30 mg/kg Intraperitoneal injection Mouse FST/TST models: Male CD-1 mice (20–25 g) were orally administered Reboxetine mesylate (5, 10, 20 mg/kg) 60 minutes before the forced swim test (6-minute session) or tail suspension test (6-minute session). Immobility time was recorded and analyzed [1][4] - Rat microdialysis model: Male Sprague-Dawley rats (250–300 g) were implanted with microdialysis probes in the prefrontal cortex. After recovery, Reboxetine mesylate (10 mg/kg, oral) was administered, and dialysates were collected every 20 minutes for 4 hours. Norepinephrine levels were quantified by HPLC [4] - Drug formulation: Reboxetine mesylate was dissolved in distilled water for oral administration; for intravenous injection, it was dissolved in physiological saline [4][5] |
| 药代性质 (ADME/PK) |
Oral bioavailability: 76% (rat, 10 mg/kg po); 85% (human, 4 mg po) [5]
- Half-life (t₁/₂): 6.2 hours (rat, iv); 12.5 hours (human, po) [5] - Volume of distribution (Vd): 2.8 L/kg (rat, iv); 3.1 L/kg (human, iv) [5] - Metabolism: Primarily metabolized in the liver via cytochrome P450 3A4 (CYP3A4) to inactive metabolites [2][5] - Excretion: 65% via urine (unchanged drug + metabolites); 25% via feces [5] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Reboxetine is not approved for marketing in the United States by the U.S. Food and Drug Administration, but is available in other countries. Limited information indicates that maternal doses of up to 10 mg daily produce low levels in milk and appear to not result in any adverse effects in breastfed infants. Until more data are available, reboxetine should be used with careful monitoring during breastfeeding. ◉ Effects in Breastfed Infants Four infants whose mothers had postpartum depression had been breastfed (extent not stated) for 1.3 to 2.1 months during maternal reboxetine therapy at an average dose of 6.5 mg (79 mcg/kg) daily. One of the mothers was also taking escitalopram 20 mg daily and another was taking sertraline 300 mg daily. None of the infants exhibited any adverse reactions. Three of the infants had normal Denver developmental scores; the fourth whose mother was taking reboxetine had a developmental age of only 71% of normal, but the problem predated maternal reboxetine therapy. Five women used reboxetine during pregnancy and lactation (extent not stated) in unspecified doses. No adverse effects were noted in their infants and normal developmental milestones were reported. ◉ Effects on Lactation and Breastmilk Reboxetine increased serum prolactin in male subjects. The relevance of this finding to nursing mothers is not clear. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. None of the mothers were taking reboxetine. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. Plasma protein binding rate: 97% (human plasma, ultrafiltration method) [5] - In vitro toxicity: CC₅₀ > 100 μM in PC12 cells and primary cortical neurons [5] - Clinical side effects: In MDD patients (4–8 mg/day, 6–8 weeks), common adverse events included dry mouth (28%), constipation (22%), insomnia (18%), and sweating (15%)[2][3] - Animal acute toxicity: LD₅₀ > 200 mg/kg (mouse, po); no mortality or obvious toxicity signs at doses up to 150 mg/kg [5] |
| 参考文献 | |
| 其他信息 |
A morpholine derivative that is a selective and potent noradrenaline reuptake inhibitor; it is used in the treatment of DEPRESSIVE DISORDER.
Reboxetine mesylate is a selective, potent, and reversible norepinephrine reuptake inhibitor (NRI) [1][5] - Its mechanism of action involves blocking NET-mediated norepinephrine reuptake into presynaptic neurons, increasing norepinephrine levels in the synaptic cleft to exert antidepressant effects [1][4] - Clinically approved for the treatment of major depressive disorder (MDD); it shows efficacy in improving mood, anhedonia, and psychomotor retardation in MDD patients [2][3] - It exhibits no significant affinity for muscarinic, histaminergic, or serotonergic receptors, minimizing anticholinergic and sedative side effects compared to tricyclic antidepressants [5] |
| 分子式 |
C20H27NO6S
|
|---|---|
| 分子量 |
409.5
|
| 精确质量 |
409.155
|
| 元素分析 |
C, 58.66; H, 6.65; N, 3.42; O, 23.44; S, 7.83
|
| CAS号 |
98769-84-7
|
| 相关CAS号 |
Reboxetine; 71620-89-8; (R,R)-Reboxetine mesylate; 105017-39-8
|
| PubChem CID |
127150
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
443.7ºC at 760 mmHg
|
| 熔点 |
170-171ºC
|
| 闪点 |
188.2ºC
|
| LogP |
4.107
|
| tPSA |
102.47
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
425
|
| 定义原子立体中心数目 |
2
|
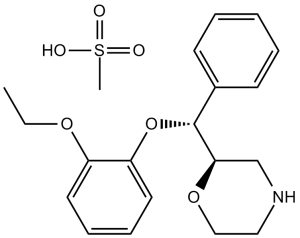
| SMILES |
S(C([H])([H])[H])(=O)(=O)O[H].O1C([H])([H])C([H])([H])N([H])C([H])([H])[C@]1([H])[C@@]([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])OC1=C([H])C([H])=C([H])C([H])=C1OC([H])([H])C([H])([H])[H]
|
| InChi Key |
CGTZMJIMMUNLQD-STYNFMPRSA-N
|
| InChi Code |
InChI=1S/C19H23NO3.CH4O3S/c1-2-21-16-10-6-7-11-17(16)23-19(15-8-4-3-5-9-15)18-14-20-12-13-22-18;1-5(2,3)4/h3-11,18-20H,2,12-14H2,1H3;1H3,(H,2,3,4)/t18-,19-;/m1./s1
|
| 化学名 |
(2R)-2-[(R)-(2-ethoxyphenoxy)-phenylmethyl]morpholine;methanesulfonic acid
|
| 别名 |
PNU 155950E; PNU155950E; PNU-155950E; FCE-20124 mesylate; PNU-155950E mesylate; FCE 20124 mesylate; PNU 155950E mesylate; FCE20124 mesylate; PNU155950E mesylate; Reboxetine mesilate; Reboxetine; Edronax; Reboxetine mesylate; Vestra (TN); AC1L2RIX; AC1Q6WCV; DSSTox_CID_25690; DSSTox_RID_81062; DSSTox_GSID_45690.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 82~125 mg/mL (200.2~305.3 mM)
Water: < 1 mg/mL Ethanol: ~82 mg/mL (~200.2 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.08 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.08 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.08 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 110 mg/mL (268.62 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4420 mL | 12.2100 mL | 24.4200 mL | |
| 5 mM | 0.4884 mL | 2.4420 mL | 4.8840 mL | |
| 10 mM | 0.2442 mL | 1.2210 mL | 2.4420 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|