| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Reboxetine is rapidly and extensively absorbed following oral administration. Multiple samples of blood and milk were obtained over a dose interval at steady-state from four women who were taking reboxetine for postnatal depression. Drug concentrations in plasma and milk were measured by high performance liquid chromatography and milk/plasma ratio (M/P), absolute infant dose and relative infant dose were estimated by standard methods. Their four, breastfed, infants were also examined clinically, and a blood sample was taken for drug analysis. The median (range) dose taken by the women was 6 (4-10) mg/day. There was no significant difference in reboxetine concentration between paired fore-and hind-milk samples. The mean (95% CI) M/P was 0.06 (0.03, 0.09). Absolute infant dose was 1.7 (0.7, 2.4) ug/kg/day for reboxetine while the relative infant dose was 2.0% (1.3, 2.7%). ... The concentrations of reboxetine in plasma from the four infants were <4 ug/L, 2.6 ug/L, 2.3 ug/L and 5 ug/L, respectively. Reboxetine is known to be excreted in breast milk. The drug appears to be distributed into total body water. Reboxetine is 97% bound to human plasma proteins in young and 92% in elderly (with affinity markedly higher for alpha1 acid glycoprotein than albumin), with no significant dependence of the concentration of drug. After oral administration of a single 4 mg reboxetine dose to healthy volunteers, peak levels of about 130 ng/mL are achieved within 2 hr post-dosing. Data indicate that absolute bioavailability is at least 60%. Reboxetine plasma levels decreased monoexponentially with a half-life of about 13 hr. Steady-state conditions are observed within 5 days. Linearity of the pharmacokinetics was shown in the range of single oral doses in the clinically recommended dose-ranges. For more Absorption, Distribution and Excretion (Complete) data for REBOXETINE (6 total), please visit the HSDB record page. Metabolism / Metabolites Reboxetine is metabolized by dealkylation, hydroxylation and oxidation followed by glucuronide or sulphate conjugation. It is metabolized by the cytochrome P450 CYP isoenzyme 3A4. The purpose of this study was to compare the disposition and the metabolic pattern of Reboxetine in several species, including man. (14)C-Reboxetine was given orally to the rat, the dog, the monkey (5 mg/kg) and man (2 and 4 mg/kg). Radioactivity was eliminated both by the renal and faecal route in the rat and the dog, mainly in urine in the monkey and man. Reboxetine was extensively metabolized. A number of urinary metabolites were quantified by radio-HPLC and tentatively identified by comparison with the retention times of reference compounds. Suggested routes of metabolic transformation are: 2-O-dealkylation; hydroxylation of the ethoxyphenoxy ring; oxidation of the morpholine ring; morpholine ring-opening; and combinations of these. Metabolites were partially or completely conjugated with glucuronic acid and/or sulphuric acid. Reboxetine is predominantly metabolized in vitro via cytochrome P4503A (CYP3A4). In vitro studies have shown that reboxetine does not inhibit the activity of the following isozymes of cytochrome P450: CYP1A2, CYP2C9, CYP2C19, and CYP2E1. Reboxetine inhibits both CYP2D6 and CYP3A4 with low binding affinities, but has shown no effect on the in vivo clearance of drugs metabolized by these enzymes. Reboxetine should be co-prescribed with caution with potent inhibitors of CYP3A4. Biological Half-Life 12.5 hours After oral administration of a single 4 mg reboxetine dose to healthy volunteers ... reboxetine plasma levels decreased monoexponentially with a half-life of about 13 hr. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Reboxetine is not approved for marketing in the United States by the U.S. Food and Drug Administration, but is available in other countries. Limited information indicates that maternal doses of up to 10 mg daily produce low levels in milk and appear to not result in any adverse effects in breastfed infants. Until more data are available, reboxetine should be used with careful monitoring during breastfeeding. ◉ Effects in Breastfed Infants Four infants whose mothers had postpartum depression had been breastfed (extent not stated) for 1.3 to 2.1 months during maternal reboxetine therapy at an average dose of 6.5 mg (79 mcg/kg) daily. One of the mothers was also taking escitalopram 20 mg daily and another was taking sertraline 300 mg daily. None of the infants exhibited any adverse reactions. Three of the infants had normal Denver developmental scores; the fourth whose mother was taking reboxetine had a developmental age of only 71% of normal, but the problem predated maternal reboxetine therapy. Five women used reboxetine during pregnancy and lactation (extent not stated) in unspecified doses. No adverse effects were noted in their infants and normal developmental milestones were reported. ◉ Effects on Lactation and Breastmilk Reboxetine increased serum prolactin in male subjects. The relevance of this finding to nursing mothers is not clear. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. None of the mothers were taking reboxetine. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. Protein Binding 98% Interactions In vitro metabolism studies indicate that reboxetine is primarily metabolised by the CYP3A4 isozyme of cytochrome P450; reboxetine is not metabolized by CYP2D6. Therefore potent inhibitors of CYP3A4 (ketoconazole, nefazodone, erythromycin and fluvoxamine), would be expected to increase plasma concentrations of reboxetine. In a study in healthy volunteers, ketoconazole, a potent inhibitor of CYP3A4, was found to increase plasma concentrations of the reboxetine enantiomers by approximately 50%. Because of reboxetine's narrow therapeutic margin, inhibition of elimination is a major concern. Reboxetine, therefore should not be given together with drugs known to inhibit CYP3A4 such as azole antifungal agents, macrolide antibiotics such as erythromycin, or fluvoxamine. Concomitant use of MAO-inhibitors and reboxetine should be avoided in view of the potential risk (tyramine-like effect) based on their mechanisms of action. Although data are not available from clinical studies, the possibility of hypokalaemia with concomitant use of potassium losing diuretics should be considered. Concomitant use of ergot derivatives and reboxetine might result in increased blood pressure. The interaction between ketoconazole and reboxetine enantiomers was studied in 11 healthy volunteers (age range, 18-47 yr) who received 4 mg of oral reboxetine on the second day of a 5-day regimen of 200 mg/day of ketoconazole or 4 mg of reboxetine alone in a crossover design. Results showed that ketoconazole increased R,R-(-)-reboxetine and S,S-(+)-reboxetine mean area under the plasma concentration-time curves (AUC) by 58 and 43%, respectively. Oral clearance of both enantiomers was subsequently decreased 34 and 24%, respectively. Ketoconazole did not significantly affect maximal plasma concentrations. Mean terminal half-life after administration of ketoconazole was significantly longer than after reboxetine alone. The AUC ratio for R,R-(-)-reboxetine to S,S-(+)-reboxetine was reduced by ketoconazole administration. |
| 参考文献 | |
| 其他信息 |
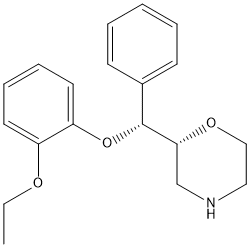
Reboxetine is an aromatic ether.
Reboxetine is an antidepressant drug used in the treatment of clinical depression, panic disorder and ADD/ADHD. Its mesylate (i.e. methanesulfonate) salt is sold under tradenames including Edronax, Norebox, Prolift, Solvex, Davedax or Vestra. Reboxetine has two chiral centers, but it only exists as two enantiomers, (R,R)-(-)- and (S,S)-(+)-reboxetine. A morpholine derivative that is a selective and potent noradrenaline reuptake inhibitor; it is used in the treatment of DEPRESSIVE DISORDER. Drug Indication For the treatment of clinical depression. Mechanism of Action Reboxetine is a selective inhibitor of noradrenaline reuptake. It inhibits noradrenaline reuptake in vitro to a similar extent to the tricyclic antidepressant desmethylimipramine. Reboxetine does not affect dopamine or serotonin reuptake and it has low in vivo and in vitro affinity for adrenergic, cholinergic, histaminergic, dopaminergic and serotonergic receptors. Reboxetine is a highly selective and potent inhibitor of noradrenaline reuptake. It has only a weak effect on the 5-HT reuptake and does not affect the uptake of dopamine. Noradrenaline reuptake inhibition and the consequent increase of noradrenaline availability in the synaptic cleft and modification of noradrenergic transmission, reportedly is among the most relevant mechanisms of action of known antidepressant drugs. Therapeutic Uses This open-label study assessed the effectiveness of reboxetine, a selective norepinephrine reuptake inhibitor, in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) resistant to a previous methylphenidate trial. Thirty-one child and adolescent outpatients, aged 8 to 18 (mean age, 11.7; SD = 2.87) years, diagnosed with ADHD were enrolled in a 6-week open-label study. Assessments included rater-administered scales (DSM-IV ADHD Scale; Clinical Global Impressions Scale), parent-administered scales (the Abbreviated Conners Rating Scale), and self-administered-scales for the evaluation of depressive (Children's Depression Inventory) and anxiety (the Revised Children's Manifest Anxiety Scale) symptoms. Reboxetine was initiated and maintained at a dose of 4 mg/day. RESULTS: A significant decrease in ADHD symptoms, on all scales measured, was noted. Adverse effects were relatively mild and transient. The most common adverse effects were drowsiness/sedation and gastrointestinal complaints. CONCLUSIONS: The results of the current open-label study suggest the effectiveness of reboxetine in the treatment of ADHD in methylphenidate-resistant children and adolescents. Double-blind, placebo-, and active comparator-controlled studies are indicated to rigorously test the efficacy of reboxetine in ADHD. /Not approved for use in the US/ Reboxetine, a potent and selective noradrenaline reuptake inhibitor, has been approved for treatment of major depression. The aim of this study was to investigate the efficacy and tolerability of reboxetine in depressive outpatients undergoing treatment in routine clinical practice. STUDY DESIGN AND METHODS: This post-marketing surveillance study was conducted to evaluate the therapeutic efficacy and tolerability of standard therapeutic doses of reboxetine in patients with depressive symptoms, particularly when administered in routine clinical practice. The 1835 patients (mean 54 years of age) evaluated showed demographic characteristics representative of the general depressive population. The majority of patients received the recommended dose of reboxetine 8 mg/day. Measures of efficacy showed improvement in depressive symptoms with reboxetine therapy over the mean observational period of 9.6 weeks. Response to therapy, defined as Hamilton depression scale 21-item version score reduction of greater or equal to 50%, was reported in 83% of patients. The effects of reboxetine were rated by physicians as 'good' or 'very good' in 86% of patients at the last visit. The tolerability of reboxetine was rated by physicians as 'good' or 'very good' in 92% of patients at all evaluations. No adverse events that were possibly related to reboxetine therapy occurred in greater than 1% of patients. The results of this study suggest that reboxetine is safe and well tolerated and may improve symptoms in depressive patients treated in routine clinical practice. /Not approved for use in the US/ /EXPL/ Although several approaches have been attempted for cocaine dependence, the pharmacological treatment of this serious disorder remains unclear. To date, desipramine, a tricyclic antidepressant of great noradrenergic activity, has shown the best results. Reboxetine, a selective noradrenaline reuptake inhibitor, might be an effective therapeutic option for this severe drug addiction. The aim was preliminarily to assess reboxetine in a group of cocaine-dependent patients ... Twenty six patients with a diagnosis of cocaine dependence disorder (DSM-IV 304.20) were selected to receive open treatment with reboxetine, 8 mg/day, for 12 weeks. Follow up assessments comprised cocaine consumption, treatment retention rate and change in standard structured psychometric instrument scores: cocaine selective severity assessment, Hamilton anxiety scale, Hamilton depression scale and clinical global impression, throughout the treatment period. Data were obtained from 20 patients; 10 of them remained abstinent, whereas the other 10 consumed cocaine at some time during the study. The treatment retention rate at week 12 was 61.5%. The psychometric instrument mean scores showed marked decreases throughout the treatment period. Reboxetine might be an effective and safe therapeutic option for cocaine dependence disorder. The aversive effects, as well as the high blockage reported by some patients consuming cocaine during the trial, might be related to treatment. Reboxetine is indicated for the acute treatment of depressive illness/major depression and for maintaining the clinical improvement in patients initially responding to treatment. /Not approved for use in the US/ Drug Warnings Reboxetine should not be used in the treatment of children and adolescents under the age of 18 years. Suicide-related behaviors (suicide attempt and suicidal thoughts), and hostility (predominantly aggression, oppositional behavior and anger) were more frequently observed in clinical trials among children and adolescents treated with antidepressants compared to those treated with placebo. If, based on clinical need, a decision to treat is nevertheless taken, the patient should be carefully monitored for the appearance of suicidal symptoms. In addition, long-term safety data in children and adolescents concerning growth, maturation and cognitive and behavioral development are lacking. Elderly patients have been studied in clinical trials at doses of 2 mg twice a day. However, safety and efficacy have not been evaluated in placebo-controlled conditions. Therefore, as for other antidepressants that have not been studied in placebo-controlled conditions, reboxetine cannot be recommended. As reboxetine has not been tested in patients with convulsive disorders in clinical studies and since rare cases of seizures have been reported in clinical studies, it should be given under close supervision to subjects with a history of convulsive disorders and it must be discontinued if the patient develops seizures. As with all antidepressants, switches to mania/hypomania have occurred during the clinical studies. Close supervision of bipolar patients is, therefore, recommended. For more Drug Warnings (Complete) data for REBOXETINE (13 total), please visit the HSDB record page. Pharmacodynamics Reboxetine is a selective noradrenaline reuptake inhibitor (NaRI), the first drug of new antidepressant class. Reboxetine is an a-ariloxybenzyl derivative of morpholine. Reboxetine is primarily used to treat depression but has also been found useful in the treatment of narcolepsy and panic disorders. |
| 分子式 |
C19H24NO3+
|
|---|---|
| 分子量 |
314.39876
|
| 精确质量 |
313.167
|
| CAS号 |
71620-89-8
|
| 相关CAS号 |
Reboxetine mesylate;98769-84-7;Reboxetine-d5 mesylate;1285918-53-7
|
| PubChem CID |
127151
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
443.7±30.0 °C at 760 mmHg
|
| 熔点 |
141-143ºC
|
| 闪点 |
188.2±14.0 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.553
|
| LogP |
2.82
|
| tPSA |
39.72
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
333
|
| 定义原子立体中心数目 |
2
|
| SMILES |
O([C@@H]([C@H]1CNCCO1)c1ccccc1)c1c(OCC)cccc1
|
| InChi Key |
CBQGYUDMJHNJBX-RTBURBONSA-N
|
| InChi Code |
InChI=1S/C19H23NO3/c1-2-21-16-10-6-7-11-17(16)23-19(15-8-4-3-5-9-15)18-14-20-12-13-22-18/h3-11,18-20H,2,12-14H2,1H3/t18-,19-/m1/s1
|
| 化学名 |
(2R)-2-[(R)-(2-ethoxyphenoxy)-phenylmethyl]morpholine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1807 mL | 15.9033 mL | 31.8066 mL | |
| 5 mM | 0.6361 mL | 3.1807 mL | 6.3613 mL | |
| 10 mM | 0.3181 mL | 1.5903 mL | 3.1807 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。