| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
p53-MDM2 (IC50 = 6 nM)
RG7388 (Idasanutlin): Proto-Oncogene Proteins c-mdm2 (MDM2)-Tumor Suppressor Protein p53 interaction (potent and selective inhibition) [1] RG7388 (Idasanutlin): Proto-Oncogene Proteins c-mdm2 (MDM2) [2] Idasanutlin (RG7388): Proto-Oncogene Proteins c-mdm2 (MDM2) (oral MDM2 antagonist, inhibits p53-MDM2 interaction) [3] |
||
|---|---|---|---|
| 体外研究 (In Vitro) |
在表达野生型 p53 的癌细胞中,isanutlin 抑制细胞增殖,IC50 为 30 nM,并剂量依赖性诱导 p53 稳定、细胞周期停滞以及细胞凋亡。 [1]
RG7388显示出改善的体外结合以及细胞效力/选择性。因此,选择化合物12进行进一步研究。在基于细胞的机制研究中),RG7388在表达野生型p53的癌症细胞中诱导剂量依赖性p53稳定、细胞周期阻滞和凋亡,这与非基因毒性p53激活机制一致。[1] RG7388诱导的SJSA骨肉瘤细胞凋亡相对于药物暴露延迟,但不需要持续治疗[2]。 1. RG7388是第二代小分子p53-MDM2抑制剂,相较于第一代抑制剂RG7112,具有更优的效价和选择性[1] 2. RG7388诱导的肿瘤细胞凋亡相较于药物暴露存在延迟效应,且无需持续给药即可触发凋亡[2] 3. Idasanutlin(RG7388)在体外实验中表现出剂量和给药方案依赖性的p53激活效应;研究评估了其抗增殖作用,发现每日给药(QD)时,MIC-1水平随药物暴露量增加而升高[3] |
||
| 体内研究 (In Vivo) |
在初步药效测试中,RG7388的每日剂量为30mg/kg,每周两次剂量为50mg/kg,在我们的肿瘤模型中具有统计学上的等效性。此外,每周给药50mg/kg相当于每天给药10mg/kg。对这些数据进行建模和模拟表明了几种可能的间歇临床给药方案。进一步的临床前分析证实了这些时间表是可行的选择。[2]
与RG7112相比,RG7388在裸鼠体内对已建立的人SJSA1骨肉瘤异种移植物也取得了令人印象深刻的体内疗效[1]。 1. RG7388在异种移植模型中可激活p53、抑制肿瘤生长并提高模型动物的存活率[3] 2. 在小鼠骨肉瘤异种移植模型中,RG7388每日30 mg/kg给药与每周两次50 mg/kg给药的抗肿瘤活性在统计学上等效;每周50 mg/kg给药也与每日10 mg/kg给药效果相当[2] 3. 基于RG7388在小鼠骨肉瘤异种移植模型中的数据构建的药代动力学-药效学(PKPD)模型预测,间歇性给药方案可实现肿瘤静止,该预测结果经后续临床前分析验证[2] |
||
| 酶活实验 |
p53-MDM2 HTRF 测定使用 50 mM Tris-HCl、pH 7.4、100 mM NaCl、1 mM DTT 和 0.02 或 0.2 mg/ml BSA 缓冲液。小分子抑制剂的等分试样作为 10 mM DMSO 的储备溶液保存在 96 深孔板中 4°C。在测试之前,将其解冻并混合。将生物素化的 p53 肽和 GST-MDM2 与该物质在 37°C 下孵育一小时。添加 Eu-8044-链霉亲和素和 Phycolink 山羊抗 GST(1 型)别藻蓝蛋白后,需要在室温下孵育一小时。使用 Envision 荧光读数器读取板。板之间一式两份或一式三份的数据集用于计算 IC50 值。 XLfit4 (Microsoft) 使用具有 4 参数 Logistic 模型的 Sigmoidal 剂量响应模型和方程 Y= (A+ ((BA)/ (1+ ((C/x)^D)))) 分析数据,其中 A B 和 B 分别是在不存在或存在无限抑制剂化合物的情况下的酶活性,C 是 IC50,D 是 Hill 系数。
|
||
| 细胞实验 |
四唑染料测定用于评估细胞增殖。浓度与抑制百分比对数图的线性回归得出细胞增殖被抑制 50% (IC50) 或 90% (IC90) 时的浓度。[1]
癌症细胞系的体外试验[2] RG7388在DMSO中以1和10 mmol/L的浓度制备,并在-20°C下等分储存。SJSA、RKO、HCT116、H460、A375、SK-MEL-5、SW480、MDA435和HeLa细胞购自ATCC。细胞系通过Promega认证服务通过短串联重复分析进行认证。对于体外研究,细胞在ATCC指定的培养基中培养。培养基中补充了10%FBS和1%200 nmol/L L-谷氨酰胺。为了评估细胞活力,在正常生长培养基中的96孔板中,以确定的最佳生长密度接种细胞,进行5天的测定。从300μmol/L开始,将RG7388的连续稀释液(在新鲜培养基中为1-3)分三次应用于孔(1-10),最终浓度范围为0.01至30μmol/L,对照孔用0.3%的DMSO处理,相当于最高RG7388浓度的DMSO。如前所述,通过MTT还原为甲赞来测量细胞呼吸,作为细胞存活率的指标。 按照Tovar及其同事的描述确定凋亡百分比。对于蛋白质印迹分析,细胞在T-75培养瓶中培养(总体积4 mL,5×105个细胞/孔),并在37°C、5%CO2下孵育过夜。细胞用0.3或1.8μmol/L的RG7388或0.1%的DMSO处理作为对照。处理时间为16小时,在RG7388洗脱前和洗脱后4、8、24和48小时制备裂解物。 1. 为评估Idasanutlin(RG7388)的抗增殖作用和p53激活效应,开展体外细胞实验检测生物标志物(如MIC-1)的表达,并评价药物在不同给药方案下的抗增殖活性;同时分析了药物暴露量与p53激活的相关性[3] 2. 为研究RG7388的凋亡诱导效应,将肿瘤细胞系与药物共处理,监测凋亡的时间进程,以明确药物暴露量与凋亡发生的关系[2] |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Pharmacokinetics [3]
\nIdasanutlin peak concentrations typically occurred 6 to 8 h after oral administration without food, and declined thereafter, with terminal half-life of ≈30 h (Fig. 2A). Exposure was approximately dose proportional after the first dose (i.e., day 1) and following repeat dosing (i.e., day 3 for QD × 3 regimens, day 5 for QD × 5 regimens), although increases appeared to be less than dose proportional at doses above 600 mg, suggesting a saturation in intestinal absorption at this dose level (Fig. 2B). However, inter-patient variability in exposure was high with all dosing regimens (Fig. 2B). Exposure was approximately twofold higher on the final day of QD × 3 and QD × 5 dosing compared with the first dose (Fig. 2B), but there was no accumulation with QW × 3 dosing (data not shown). For a specified daily dose, cumulative idasanutlin exposure over the whole 28-day dosing cycle was greatest with a QD × 5 regimen, reflecting the higher total dose administered (i.e., 5 days of dosing vs. 3 days of dosing or 3 single doses).\n \nFood effects on idasanutlin pharmacokinetics [3] \nTen patients received 800-mg doses of idasanutlin, either with a high-fat/high-calorie meal or while fasted. Dosing employed a half-replicate, crossover design, which resulted in 15 pairs of fed versus fasted data from the 10 patients. On average, idasanutlin exposure was higher when taken with food (mean maximum plasma concentration was 14% higher and area under the curve extrapolated to infinity was 43% higher), but variability was high and as the 90% confidence intervals encompassed unity, it was concluded that food had no clinically meaningful effect on idasanutlin exposure (Online Resource Table S7; Fig. 2C).\n \nPharmacodynamic analysis [3] \nAfter idasanutlin dosing, circulating macrophage inhibitory cytokine 1 (MIC-1) levels increased generally in a dose-exposure–dependent manner (Fig. 2D). Consequently, trends in MIC-1 responses to treatment mirrored trends in idasanutlin exposure, as described in Population PK/PD Analysis section.\n \nSixteen of the 31 patients (51.6%) evaluated by positron emission tomography analysis for changes in tumor proliferation rates with idasanutlin treatment achieved a partial proliferative response as their best percentage maximum standardized uptake value change from baseline during cycle 1, indicating a decrease of ≥ 25% (Online Resource Fig. S2).\n \nPopulation PK/PD analysis [3] \nSimulations with the indirect PK/MIC-1 model (Online Resource Supplementary Methods) indicated that despite some high variability, the release of MIC-1 following idasanutlin treatment is concentration dependent; the higher the idasanutlin concentrations, the higher the release of MIC-1. Weekly dosing with idasanutlin resulted in lower maximum release but a more sustainable effect on MIC-1 over the 28-day treatment cycle compared with a daily regimen (for the same level of dose) (Fig. 3).\n\n \n\nIdasanutlin (RG7388) is a MDM2 (murine double minute 2) inhibitor that is being investigated for its anticancer effects, particularly in tumors with wild-type TP53 (e.g., acute myeloid leukemia, solid tumors). Here’s an overview of its pharmacokinetics (PK) based on available preclinical and clinical data:\n \n1. Absorption \nRoute of Administration: Oral (tablet formulation). \n\nBioavailability: Limited data, but oral absorption is moderate (~30-50% in preclinical models).\n \nFood Effect: High-fat meals may significantly increase absorption (observed in clinical trials). Thus, it is often administered with food to enhance bioavailability.\n \n2. Distribution \nProtein Binding: Highly protein-bound (>99%, primarily to albumin).\n \nVolume of Distribution (Vd): Not well characterized, but likely moderate due to high protein binding.\n \nTissue Penetration: Preclinical data suggest distribution into tumor tissues, but CNS penetration is likely limited.\n \n3. Metabolism \nPrimary Pathway: Hepatic metabolism, primarily via CYP3A4 (major) and UGT1A1/UGT1A3 (glucuronidation).\n \nMetabolites: Several oxidative and conjugated metabolites (mostly inactive or weakly active).\n \nDrug-Drug Interactions (DDIs):\n \nCYP3A4 inducers (e.g., rifampin) → May decrease idasanutlin exposure.\n \nCYP3A4 inhibitors (e.g., ketoconazole) → May increase idasanutlin exposure.\n \nUGT inhibitors (e.g., atazanavir) → Potential increase in exposure.\n \n4. Elimination \nHalf-life (t½): ~4–8 hours (variable between patients).\n \nClearance: Primarily hepatic with biliary excretion.\n \nExcretion: Mostly fecal (~70-80%), with minimal renal excretion (<5%).\n \n5. Pharmacokinetic Variability \nInterpatient Variability: High, possibly due to differences in CYP3A4/UGT activity, food effects, and protein binding.\n \nDose Proportionality: Nonlinear PK at higher doses (saturation of absorption or metabolism).\n \n6. Special Populations \nHepatic Impairment: Expected to significantly increase exposure (not well studied).\n \nRenal Impairment: Unlikely to have a major effect (minimal renal excretion).\n \nPediatric/Elderly: Limited data; studies are ongoing.\n \n7. Clinical Implications \nOptimal Dosing: Typically given once daily or intermittently (e.g., 5 days on/2 days off) to manage toxicity (e.g., gastrointestinal effects, thrombocytopenia).\n \nTherapeutic Drug Monitoring (TDM): May be useful due to high PK variability.\n \nSummary Table of Idasanutlin PK \nParameter\tCharacteristics \nRoute\tOral (with food) \nBioavailability\tModerate (~30-50%) \nProtein Binding\t>99% (albumin) \nMetabolism\tCYP3A4, UGT1A1/1A3 \nHalf-life\t~4–8 hours \nExcretion\tFeces (~70-80%), urine (<5%) \nKey DDIs\tCYP3A4 inducers/inhibitors \nOngoing Research \nClinical trials continue to refine the PK profile, particularly in combination therapies (e.g., with venetoclax in AML). 1. Idasanutlin (RG7388) exposure was approximately dose-proportional at low doses, but less than dose-proportional at doses > 600 mg; inter-patient variability in exposure was high across all dosing regimens [3] 2. The cumulative exposure of Idasanutlin (RG7388) over a 28-day cycle was the highest with the QD × 5 regimen [3] |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and tolerability [3]
\nThe median duration of treatment for all patients was 36 days (range, 1–726 days), with 15 patients (15.2%) treated for > 91 days (Online Resource Table S3). The median (range) number of total daily doses received in the QW × 3, QD × 3, and QD × 5 cohorts, respectively, was 10.5 (2–72), 9.0 (6–42), and 10.0 (1–130). All 99 patients comprised the safety population; across all cohorts, 78 patients (78.8%) received ≤ 2 treatment cycles.\n \nThe MTD for QW × 3 dosing was 3200 mg (given as 1600 mg twice daily [BID]), with DLTs of nausea, thrombocytopenia, and vomiting (Online Resource Table S4), all reported at a total daily dose of 1600 mg or higher. The MTD for QD × 3 dosing was 1000 mg (given as 500 mg BID), with DLTs of thrombocytopenia, febrile neutropenia, neutropenia, and pancytopenia. For QD × 5 dosing, the MTD was 500 mg (given QD), with DLTs of thrombocytopenia, neutropenia, febrile neutropenia, and diarrhea. A total of 31 DLTs were reported across all cohorts (n = 99), with 21 patients (21.2%; dose-escalation cohorts, n = 20; apoptosis cohort, n = 1) having ≥ 1 DLT. The most common DLT was thrombocytopenia, occurring in 16 of 99 patients (16.2%). Other DLTs included neutropenia (5 [5.1%]), febrile neutropenia (3 [3.0%]), nausea (2 [2.0%]), as well as leukopenia, pancytopenia, diarrhea, and vomiting (1 each [1.0%]). Within the dose-escalation cohorts, DLTs were more common in patients on daily (40% for QD × 3 and 32.4% for QD × 5) versus weekly dosing schedules (8.3%), with a higher incidence of DLTs related to hematologic and lymphatic system disorders reported with daily regimens (Online Resource Table S4).\n \nAll 99 patients experienced ≥ 1 AE that was considered by the investigator to be related to study treatment (Table 1). The most common treatment-related AEs were diarrhea (74.7%), nausea (71.7%), vomiting (50.5%), decreased appetite (43.4%), and thrombocytopenia (39.4%; Online Resource Table S5). In general, treatment-related AEs occurred at the highest frequencies with the QD × 3 schedule; the lowest frequencies were observed with the QD × 5 schedule (Online Resource Table S5).\n \nGrade ≥ 3 AEs of any cause occurred in 63 patients (63.6%) and were reported in higher incidences in the QD dosing regimens (Table 1). The most common grade ≥ 3 any-cause AEs were thrombocytopenia (29.3%), anemia (20.2%), neutropenia (16.2%), nausea (11.1%), and diarrhea (7.1%). Serious AEs (SAEs) were reported in 32 patients (32.3%) across all study groups (Table 1; Online Resource Table S6). Treatment-related SAEs were reported in 25 patients (25.3%); the most frequently reported (in 24 of 25 patients) were related to blood and lymphatic system disorders: thrombocytopenia/decreased platelet count (14 events), febrile neutropenia (5 events), neutropenia/decreased neutrophil count (4 events), leukopenia/decreased white blood cell count (3 events), and anemia (2 events). Treatment-related SAEs were more frequently reported with QD dosing (QD × 3, 7 of 15 [46.7%]; QD × 5, 13 of 34 [38.2%]) than QW × 3 dosing (4 of 36 [11.1%]) (Online Resource Table S6).\n \nThe majority of patients (81 of 99 [81.8%]) discontinued treatment due to non-safety reasons: disease progression (n = 77), patient consent withdrawal (n = 3), and other reason unspecified (n = 1). Eighteen patients (18.2%) withdrew due to AEs, 8 of which were considered SAEs. More AE-related discontinuations occurred in patients receiving daily dosing regimens, excluding the apoptosis imaging cohort (QD × 3, 20.0%; QD × 5, 29.4%), compared with those receiving QW × 3, excluding the food effect cohort (11.1%). The most common AEs resulting in study drug discontinuation among all patients were neutropenia, thrombocytopenia, and pulmonary embolism (3.0% each). AEs associated with study withdrawal were more likely to be hematologic in nature and grade ≥ 3 in severity.\n \nDose modifications/interruptions due to an AE were reported in 44 patients (44.4%) and occurred at similar frequencies with the weekly and daily schedules (Table 1). This included 16 of 36 patients (44.4%) on QW × 3 dosing, while 7 of 15 patients (46.7%) on QD × 3 dosing and 18 of 34 (52.9%) on QD × 5 dosing required dose modifications. The most frequently reported AEs leading to dose modification were thrombocytopenia (24.2%) and neutropenia (9.1%).\n \nOverall, 7 deaths occurred during treatment or over the 28 days following the last study dose: 5 were due to progressive disease and 2 were due to an SAE (Table 1). One death (QW × 3 cohort) was due to an intra-abdominal hemorrhage and pulmonary embolism, both determined to be unrelated to study treatment. The other death (QD × 5 cohort) was due to pulmonary embolism and possibly related to study treatment. 1. The most common adverse events of Idasanutlin (RG7388) in patients with advanced malignancies were diarrhea, nausea/vomiting, decreased appetite, and thrombocytopenia [3] 2. Dose-limiting toxicities of Idasanutlin (RG7388) included nausea/vomiting and myelosuppression; myelosuppression was more frequent with QD dosing and correlated with pharmacokinetic exposure [3] 3. Grade ≥ 3 neutropenia (absolute neutrophil count < 1 × 10⁹ cells/L) and grade ≥ 3 thrombocytopenia (platelet count < 50 × 10⁹ cells/L) during the first treatment cycle were associated with the 28-day cumulative area under the curve (AUC₀-28d) of Idasanutlin (RG7388) [3] |
||
| 参考文献 |
|
||
| 其他信息 |
Idasanutlin has been used in trials studying the treatment of Neoplasms, Non-Hodgkin's Lymphoma, Leukemia, Myeloid, Acute, Recurrent Plasma Cell Myeloma, and Neoplasms, Leukemia, Acute Myeloid Leukemia.
Idasanutlin is an orally available, small molecule, antagonist of MDM2 (mouse double minute 2; Mdm2 p53 binding protein homolog), with potential antineoplastic activity. Idasanutlin binds to MDM2 blocking the interaction between the MDM2 protein and the transcriptional activation domain of the tumor suppressor protein p53. By preventing the MDM2-p53 interaction, p53 is not enzymatically degraded and the transcriptional activity of p53 is restored. This may lead to p53-mediated induction of tumor cell apoptosis. MDM2, a zinc finger nuclear phosphoprotein and negative regulator of the p53 pathway, is often overexpressed in cancer cells and has been implicated in cancer cell proliferation and survival. Drug Indication Treatment of all conditions included in the category of malignant neoplasms (except nervous system, haematopoietic and lymphoid tissue) Treatment of acute lymphoblastic leukaemia, Treatment of acute myeloid leukaemia \n\nRestoration of p53 activity by inhibition of the p53-MDM2 interaction has been considered an attractive approach for cancer treatment. However, the hydrophobic protein-protein interaction surface represents a significant challenge for the development of small-molecule inhibitors with desirable pharmacological profiles. RG7112 was the first small-molecule p53-MDM2 inhibitor in clinical development. Here, we report the discovery and characterization of a second generation clinical MDM2 inhibitor, RG7388, with superior potency and selectivity.[1] \n\nPurpose: Antitumor clinical activity has been demonstrated for the MDM2 antagonist RG7112, but patient tolerability for the necessary daily dosing was poor. Here, utilizing RG7388, a second-generation nutlin with superior selectivity and potency, we determine the feasibility of intermittent dosing to guide the selection of initial phase I scheduling regimens.\n\nExperimental design: A pharmacokinetic-pharmacodynamic (PKPD) model was developed on the basis of preclinical data to determine alternative dosing schedule requirements for optimal RG7388-induced antitumor activity. This PKPD model was used to investigate the pharmacokinetics of RG7388 linked to the time-course of the antitumor effect in an osteosarcoma xenograft model in mice. These data were used to prospectively predict intermittent and continuous dosing regimens, resulting in tumor stasis in the same model system.\n\nResults: RG7388-induced apoptosis was delayed relative to drug exposure with continuous treatment not required. In initial efficacy testing, daily dosing at 30 mg/kg and twice a week dosing at 50 mg/kg of RG7388 were statistically equivalent in our tumor model. In addition, weekly dosing of 50 mg/kg was equivalent to 10 mg/kg given daily. The implementation of modeling and simulation on these data suggested several possible intermittent clinical dosing schedules. Further preclinical analyses confirmed these schedules as viable options.\n\nConclusion: Besides chronic administration, antitumor activity can be achieved with intermittent schedules of RG7388, as predicted through modeling and simulation. These alternative regimens may potentially ameliorate tolerability issues seen with chronic administration of RG7112, while providing clinical benefit. Thus, both weekly (qw) and daily for five days (5 d on/23 off, qd) schedules were selected for RG7388 clinical testing.[2]\n \nAim The oral MDM2 antagonist idasanutlin inhibits the p53-MDM2 interaction, enabling p53 activation, tumor growth inhibition, and increased survival in xenograft models. Methods We conducted a Phase I study of idasanutlin (microprecipitate bulk powder formulation) to determine the maximum tolerated dose (MTD), safety, pharmacokinetics, pharmacodynamics, food effect, and clinical activity in patients with advanced malignancies. Schedules investigated were once weekly for 3 weeks (QW × 3), once daily for 3 days (QD × 3), or QD × 5 every 28 days. We also analyzed p53 activation and the anti-proliferative effects of idasanutlin. Results The dose-escalation phase included 85 patients (QW × 3, n = 36; QD × 3, n = 15; QD × 5, n = 34). Daily MTD was 3200 mg (QW × 3), 1000 mg (QD × 3), and 500 mg (QD × 5). Most common adverse events were diarrhea, nausea/vomiting, decreased appetite, and thrombocytopenia. Dose-limiting toxicities were nausea/vomiting and myelosuppression; myelosuppression was more frequent with QD dosing and associated with pharmacokinetic exposure. Idasanutlin exposure was approximately dose proportional at low doses, but less than dose proportional at > 600 mg. Although inter-patient variability in exposure was high with all regimens, cumulative idasanutlin exposure over the whole 28-day cycle was greatest with a QD × 5 regimen. No major food effect on pharmacokinetic exposure occurred. MIC-1 levels were higher with QD dosing, increasing in an exposure-dependent manner. Best response was stable disease in 30.6% of patients, prolonged (> 600 days) in 2 patients with sarcoma. Conclusions Idasanutlin demonstrated dose- and schedule-dependent p53 activation with durable disease stabilization in some patients. Based on these findings, the QD × 5 schedule was selected for further development. [3] 1. RG7388 is a second-generation clinical MDM2 inhibitor, following RG7112 (the first small-molecule p53-MDM2 inhibitor in clinical development), and has superior potency and selectivity [1] 2. Restoring p53 activity by inhibiting the p53-MDM2 interaction is a promising strategy for cancer treatment, but the hydrophobic nature of the p53-MDM2 protein-protein interaction surface poses a major challenge for developing small-molecule inhibitors with favorable pharmacological profiles [1] 3. Modeling and simulation of RG7388 preclinical data identified several potential intermittent clinical dosing schedules, which were validated by further preclinical studies; weekly (qw) and daily for five days (5 d on/23 off, qd) schedules were selected for RG7388 clinical testing [2] 4. Idasanutlin (RG7388) was formulated as a microprecipitate bulk powder for oral administration in its Phase I study; the maximum tolerated dose (MTD) was 3200 mg for QW × 3, 1000 mg for QD × 3, and 500 mg for QD × 5 [3] 5. In the Phase I study of Idasanutlin (RG7388), 30.6% of patients with advanced tumors achieved stable disease, with 2 sarcoma patients showing prolonged stable disease (> 600 days); the QD × 5 schedule was selected for further clinical development [3] 6. The Phase I study of Idasanutlin (RG7388) was registered on ClinicalTrials.gov (identifier: NCT01462175) on October 31, 2011 [3] |
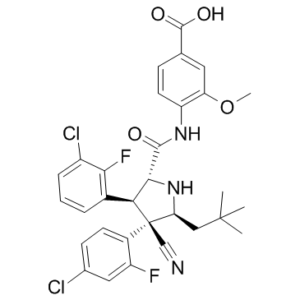
| 分子式 |
C31H29CL2F2N3O4
|
|---|---|
| 分子量 |
616.48
|
| 精确质量 |
615.15
|
| 元素分析 |
C, 60.40; H, 4.74; Cl, 11.50; F, 6.16; N, 6.82; O, 10.38
|
| CAS号 |
1229705-06-9
|
| 相关CAS号 |
Idasanutlin-d3-1;Idasanutlin (enantiomer)
|
| PubChem CID |
53358942
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
737.3±60.0 °C at 760 mmHg
|
| 闪点 |
399.7±32.9 °C
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
| 折射率 |
1.623
|
| LogP |
7.09
|
| tPSA |
114.94
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1040
|
| 定义原子立体中心数目 |
4
|
| SMILES |
ClC1=C([H])C([H])=C([H])C(=C1F)[C@@]1([H])[C@]([H])(C(N([H])C2C([H])=C([H])C(C(=O)O[H])=C([H])C=2OC([H])([H])[H])=O)N([H])[C@@]([H])(C([H])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])[C@]1(C#N)C1C([H])=C([H])C(=C([H])C=1F)Cl
|
| InChi Key |
TVTXCJFHQKSQQM-LJQIRTBHSA-N
|
| InChi Code |
InChI=1S/C31H29Cl2F2N3O4/c1-30(2,3)14-24-31(15-36,19-10-9-17(32)13-21(19)34)25(18-6-5-7-20(33)26(18)35)27(38-24)28(39)37-22-11-8-16(29(40)41)12-23(22)42-4/h5-13,24-25,27,38H,14H2,1-4H3,(H,37,39)(H,40,41)/t24-,25-,27+,31-/m0/s1
|
| 化学名 |
4-[[(2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethylpropyl)pyrrolidine-2-carbonyl]amino]-3-methoxybenzoic acid
|
| 别名 |
Idasanutlin; RG-7388; RO-5503781; RG 7388; RO5503781; 1229705-06-9; RG7388; RG-7388; Idasanutlin (RG-7388); Idasanutlin (RG7388); RO5503781; 4-((2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-neopentylpyrrolidine-2-carboxamido)-3-methoxybenzoic acid; RG7388; RO 5503781
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.06 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: 5% DMSO+40% PEG 300+5% Tween 80+ddH2O: 1.25mg/mL View More
配方 3 中的溶解度: 10 mg/mL (16.22 mM) in 0.5%HPMC 1%Tween80 (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6221 mL | 8.1106 mL | 16.2211 mL | |
| 5 mM | 0.3244 mL | 1.6221 mL | 3.2442 mL | |
| 10 mM | 0.1622 mL | 0.8111 mL | 1.6221 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
NCT Neuro Master Match - N²M² (NOA-20)
CTID: NCT03158389
Phase: Phase 1/Phase 2 Status: Completed
Date: 2023-09-28
---------
A PHASE Ib/II STUDY EVALUATING THE SAFETY AND EFFICACY OF OBINUTUZUMAB IN COMBINATION WITH IDASANUTLIN IN PATIENTS WITH RELAPSED OR REFRACTORY FOLLICULAR LYMPHOMA AND OBINUTUZUMAB OR RITUXIMAB IN COMBINATION WIT IDASANUTLIN IN PATIENTS WITH RELAPSED OR REFRACTORY DIFFUSE LARGE B-CELL LYMPHOMA .
CTID: null
Phase: Phase 1, Phase 2 Status: Completed
Date: 2016-05-12
|
|