| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
sGC
|
|---|---|
| 体外研究 (In Vitro) |
Riocigua 通过不依赖 NO 但依赖血红素的机制,以 0.1 至 100 μM 浓度依赖性地刺激重组 sGC,效果达到 2 倍至 73 倍[1]。利奥西呱会抑制洗涤血小板中的血小板功能,但不会抑制全血中的血小板功能,并且对心肌细胞的收缩和舒张没有直接影响[2]。
|
| 体内研究 (In Vivo) |
在长期治疗缺氧小鼠和注射 MCT 的大鼠中,利奥西呱(10 mg/kg/d,口服)可部分逆转肺动脉高压、右心肥厚和肺血管结构重塑[1]。
|
| 动物实验 |
Mice: Four groups of mice are used for the chronic intervention studies: ten control mice exposed to normoxic gas for 35 days; ten hypoxic gas exposed for 21 days; ten mice exposed for 35 days and given the vehicle (2% methylcellulose solution) from day 21 to day 35; and ten mice exposed for 35 days and given BAY 63-2521 (10 mg/kg) once daily by oral application from day 21 to day 35. In order to perform continuous radiotelemetry measurements of cardiac frequency and Prvs, a different group of mice is given oral application of BAY 63-2521 (10 mg/kg) once daily from day 21 to day 35 after being exposed to hypoxic gas for 35 days. Further two groups of animals are studied: control mice (n = 12) and animals exposed to hypoxia for 21 days (n = 12) in order to examine vascular reactivity in isolated mouse lungs.
Rats: One week following MCT injection, rats are randomly assigned to receive chronic BAY 63-2521 treatment. A vehicle (2% methylcellulose solution) or BAY 63-2521 (10 mg/kg) are administered orally to rats in the experimental groups once daily. On day 35, rats undergo histological evaluation and are monitored every day for the duration of their lives.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The pharmacokinetics of riociguant are dose proportional from 0.5 mg to 2.5 mg. The absolute bioavailability is approximately 94%. After oral administration, peak plasma concentrations were achieved within 1.5 hours. Food does not affect the bioavailability of riociguat. Riociguat is eliminated in the urine (40%) and feces (53%), largely as metabolites. Volume of distribution at steady state = 30 L Metabolism / Metabolites The active metabolite (M1) of riociguat is 1/3 to 1/10 as potent as riociguat. Biological Half-Life About 12 hours in patients and 7 hours in healthy subjects. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration studies, riociguat was not associated with serum enzyme elevations or with episodes of clinically apparent liver injury. Since approval of riociguat, there have been no published reports of hepatotoxicity, and the product label does not mention liver injury as an adverse event. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of riociguat during breastfeeding. The manufacturer recommends that breastfeeding be avoided during riociguat use. The drug should be absent from breastmilk 3 days after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 95% with serum albumin and alpha-1–acidic glycoprotein being the main binding components. |
| 参考文献 |
|
| 其他信息 |
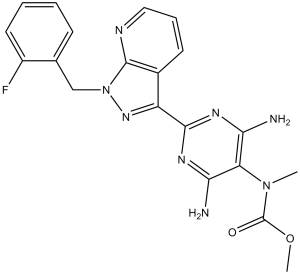
Riociguat is a carbamate ester that is the methyl ester of {4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}methylcarbamic acid. It is used for treatment of chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension It has a role as a soluble guanylate cyclase activator and an antihypertensive agent. It is a pyrazolopyridine, an aminopyrimidine, an organofluorine compound and a carbamate ester.
Riociguat is a soluble guanylate cyclase (sGC) agonist approved in the USA, Europe and several other regions for patients with group I PAH (pulmonary arterial hypertension) in WHO FC II or III; and for the treatment of patients with inoperable CTEPH (chronic thromboembolic pulmonary hypertension), or persistent/recurrent PH (pulmonary hypertension) after pulmonary endarterectomy in WHO FC II or III. Riociguat is marketed under the brand Adempas® by Bayer HealthCare Pharmaceuticals. Treatment with riociguat costs USD $7,500 for 30 days of treatment. Riociguat is a Soluble Guanylate Cyclase Stimulator. The mechanism of action of riociguat is as a Guanylate Cyclase Stimulator. Riociguat is a stimulator of guanylate cyclase which causes relaxation of vascular smooth muscle and is used to treat severe pulmonary arterial hypertension. Riociguat has not been linked to significant serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury. Drug Indication Riociguat is indicated for the treatment of adults with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH), (WHO Group 4) after surgical treatment, or inoperable CTEPH, to improve exercise capacity and WHO functional class. Riociguat is indicated for the treatment of adults with pulmonary arterial hypertension (PAH), (WHO Group 1), to improve exercise capacity, WHO functional class and to delay clinical worsening. Efficacy was shown in patients on Riociguat monotherapy or in combination with endothelin receptor antagonists or prostanoids. Studies establishing effectiveness included predominately patients with WHO functional class II–III and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (25%). FDA Label Chronic thromboembolic pulmonary hypertension (CTEPH)Adempas is indicated for the treatment of adult patients with WHO Functional Class (FC) II to III withinoperable CTEPH,persistent or recurrent CTEPH after surgical treatment,to improve exercise capacity. Pulmonary arterial hypertension (PAH)AdultsAdempas, as monotherapy or in combination with endothelin receptor antagonists, is indicated for the treatment of adult patients with pulmonary arterial hypertension (PAH) with WHO Functional Class (FC) II to III to improve exercise capacity. Efficacy has been shown in a PAH population including aetiologies of idiopathic or heritable PAH or PAH associated with connective tissue disease. PaediatricsAdempas is indicated for the treatment of PAH in paediatric patients aged less than 18 years of age and body weight ⥠50 kg with WHO Functional Class (FC) II to III in combination with endothelin receptor antagonists.  Treatment of pulmonary hypertension Mechanism of Action Riociguat is a stimulator of soluble guanylate cyclase (sGC), an enzyme in the cardiopulmonary system and the receptor for nitric oxide (NO). When NO binds to sGC, the enzyme catalyzes synthesis of the signaling molecule cyclic guanosine monophosphate (cGMP). Intracellular cGMP plays an important role in regulating processes that influence vascular tone, proliferation, fibrosis and inflammation. Pulmonary hypertension is associated with endothelial dysfunction, impaired synthesis of nitric oxide and insufficient stimulation of the NO-sGC-cGMP pathway. Riociguat has a dual mode of action. It sensitizes sGC to endogenous NO by stabilizing the NO-sGC binding. Riociguat also directly stimulates sGC via a different binding site, independently of NO. Riociguat stimulates the NO-sGC-cGMP pathway and leads to increased generation of cGMP with subsequent vasodilation. |
| 分子式 |
C20H19FN8O2
|
|---|---|
| 分子量 |
422.42
|
| 精确质量 |
422.161
|
| 元素分析 |
C, 56.87; H, 4.53; F, 4.50; N, 26.53; O, 7.58
|
| CAS号 |
625115-55-1
|
| 相关CAS号 |
Riociguat-13C,d6
|
| PubChem CID |
11304743
|
| 外观&性状 |
Light yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
567.2±50.0 °C at 760 mmHg
|
| 闪点 |
296.8±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.720
|
| LogP |
-0.31
|
| tPSA |
138.8
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
618
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC1=CC=CC=C1CN2C3=NC=CC=C3C(C4=NC(N)=C(C(N)=N4)N(C)C(OC)=O)=N2
|
| InChi Key |
WXXSNCNJFUAIDG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H19FN8O2/c1-28(20(30)31-2)15-16(22)25-18(26-17(15)23)14-12-7-5-9-24-19(12)29(27-14)10-11-6-3-4-8-13(11)21/h3-9H,10H2,1-2H3,(H4,22,23,25,26)
|
| 化学名 |
methyl N-[4,6-diamino-2-[1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]-N-methylcarbamate
|
| 别名 |
Riociguat; BAY 63-2521; BAY63-2521; BAY632521; Trade name: Adempas
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.92 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.92 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.92 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3673 mL | 11.8366 mL | 23.6731 mL | |
| 5 mM | 0.4735 mL | 2.3673 mL | 4.7346 mL | |
| 10 mM | 0.2367 mL | 1.1837 mL | 2.3673 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Long-term Extension Study of Riociguat in Patients With Symptomatic Pulmonary Arterial Hypertension.
CTID: NCT02759419
Phase: Phase 4 Status: Recruiting
Date: 2024-09-19
 Riociguat prevents hyperoxia-ablated vascular development.PLoS One. 2018 Jul 10;13(7):e0199927. |
|---|
 Riociguat decreases hyperoxia-induced vascular remodeling.PLoS One. 2018 Jul 10;13(7):e0199927. |
 Riociguat reduces and alters hyperoxia-induced lung inflammation.PLoS One. 2018 Jul 10;13(7):e0199927. |