| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
5-HT2A ( Ki = 0.17 nM ); α2c-adrenergic receptor ( Ki = 1.3 nM ); α2c-adrenergic receptor ( Ki = 1.3 nM ); D2 receptor ( Ki = 3.57 nM ); D3 receptor ( Ki = 3.6 nM ); D2L Receptor ( Ki = 4.16 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:利培酮/Risperidone与 DA 和血清素 (5HT) 受体结合,特别是在纹状体和边缘结构的神经元中。利培酮显着影响脑神经生长因子 (NGF) 水平,表明它影响内源性生长因子的周转。利培酮显着降低额叶皮层、枕叶皮层和海马体中的 BDNF 浓度,并减少或增加特定大脑结构中的 TrkB 受体。利培酮显着增加大鼠前脑区域内侧前额皮质中 D(2) 的结合达 34%。 Risperidone 对大鼠前脑区域的 CPU (37%)、NAc (32%) 和 HIP (37%) 中的 D(4) 受体产生更大的上调。 Risperidone 显着抑制激活的小胶质细胞产生 NO 和促炎细胞因子。 Risperidone (1-50 mM) 通过抑制 P-gp 活性,显着增强 Caco-2 细胞中 Rh123 的细胞内积累,IC(50) 值为 5.87 mM。
小胶质细胞最近被认为是通过在中枢神经系统(CNS)中释放促炎细胞因子、一氧化氮(NO)和活性氧(ROS)来介导神经炎症的介质。因此,据报道,小胶质细胞在阿尔茨海默病(AD)和帕金森病(PD)等神经退行性疾病的病理学中起着重要作用。精神分裂症的病理机制尚不清楚,而最近的一些神经影像学研究表明,即使是精神分裂症也可能是一种神经退行性疾病。据报道,Risperidone/利培酮可以减少精神分裂症临床过程中MRI体积的减少。最近的许多研究表明,干扰素(IFN)-γ和细胞因子等免疫机制可能与精神分裂症的病理生理学有关。在本研究中,我们分别使用Griess试验、Western印迹和ELISA研究了利培酮对IFN-γ激活的小胶质细胞产生一氧化氮、诱导型一氧化氮合酶(iNOS)表达和炎性细胞因子白细胞介素(IL)-1β、IL-6和肿瘤坏死因子(TNF)-α的影响。与氟哌啶醇相比,利培酮显著抑制了活化小胶质细胞产生NO和促炎细胞因子。利培酮处理的细胞的iNOS水平远低于氟哌啶醇处理的细胞。抗精神病药物,尤其是利培酮,可能通过抑制小胶质细胞活化而具有抗炎作用,小胶质细胞不仅对神经元有直接毒性,而且对神经发生和少突胶质细胞发生也有抑制作用,据报道,这两者在精神分裂症的病理学中都起着至关重要的作用。[3] 利培酮/Risperidone(RSP)及其主要活性代谢产物9-羟基利培酮(帕利哌酮,PALI)是药物转运蛋白P-糖蛋白(P-gp)的底物。本研究的目的是研究RSP和PALI对P-糖蛋白介导的转运的体外影响。在LLC-PK1/MDR1细胞中检测罗丹明123(Rh123)和阿霉素(DOX)的细胞内积累,以评估RSP和PALI对P-gp的抑制作用。这两种化合物都以浓度依赖的方式显著增加了Rh123和DOX的细胞内积累。RSP抑制P-gp介导的Rh123和DOX转运的IC50值分别为63.26和15.78 μM,而PALI的IC50值>100 μM,表明PALI是一种效力较低的P-gp抑制剂。利用Caco-2和原代培养的大鼠脑微血管内皮细胞(RBMECs)研究RSP对作为P-gp底物的联合用药的肠道吸收和血脑屏障(BBB)转运的可能影响。RSP,1–50 μM,通过抑制P-gp活性显著增强Caco-2细胞中Rh123的细胞内积累,IC50值为5.87 μM.暴露于10 μM RSP,Rh123在Caco-2和RBMECs单层上的表观渗透系数在顶端到基底外侧方向上分别增加到2.02和2.63倍,但在基底外侧到顶端方向上分别降低到0.37和0.21倍。这些数据表明,RSP和PALI在较小程度上有可能通过抑制P-gp介导的转运来影响药物动力学,从而影响联合用药的药效学。然而,目前还没有解决这个问题的人类数据。特别是,RSP可能通过抑制P-gp介导的PALI穿过BBB内皮细胞的流出来促进其脑浓度,从而与其自身的活性代谢产物PALI相互作用[4]。 |
| 体内研究 (In Vivo) |
尽管雄性大鼠的催乳素和皮质酮显着升高,但利培酮不会显着影响体重增加(BWG)、食物摄入量(FI)、葡萄糖耐量或瘦素水平。利培酮显着增加雌性大鼠的 BWG 和 FI。利培酮 (0.05 mg/kg) 会增加白色脂肪组织 (WAT) 中的食物摄入量和瘦素基因表达,但大鼠体重增加率不受影响。利培酮 (0.5 mg/kg) 会导致大鼠体重增加减少,并增强 BAT 中的 Ucp1 基因表达和血清催乳素浓度。
抗精神病药物氟哌啶醇和利培酮/Risperidone广泛用于治疗精神分裂症。前一种药物主要作用于多巴胺(DA)D(2)受体,而利培酮结合DA和血清素(5HT)受体,特别是在纹状体和边缘结构的神经元中。最近的证据表明,神经营养因子也可能参与中枢神经系统(CNS)的抗精神病作用。我们之前报道过氟哌啶醇和利培酮显著影响脑神经生长因子(NGF)水平,表明这些药物会影响内源性生长因子的周转。脑源性神经营养因子(BDNF)支持发育和成熟脑DA神经元的存活和分化。我们假设氟哌啶醇或利培酮治疗会影响脑BDNF的合成/释放,并通过在食物中添加氟哌啶醇和利培酮治疗29天后测量大鼠脑区的BDNF和TrkB来验证这一假设。药物治疗对大脑区域的重量没有影响。然而,长期服用这些药物会改变脑内BDNF的合成或释放以及TrkB免疫反应性的表达。氟哌啶醇和利培酮均显著降低了额叶皮层、枕叶皮层和海马中的BDNF浓度,并降低或增加了选定脑结构中的TrkB受体。由于BDNF可以作用于多种中枢神经系统神经元,因此有理由假设这种神经营养因子脑水平的改变可能构成抗精神病药物的作用机制之一。这些观察结果也支持了神经营养因子在精神分裂症患者脑功能改变中发挥作用的可能性。[1] 通过定量体外受体放射自显影比较了非典型抗精神病药物奥氮平、利培酮Risperidone/和喹硫平长期治疗(28天)后大鼠前脑区域多巴胺(DA)D(1)样(D(1,D(5))和D(2)样(D2,D(3),D(4))受体家族成员的变化。奥氮平和利培酮显著增加了内侧前额叶皮层(MPC;67%和34%)、尾壳核(CPu;平均42%,25%)、伏隔核(NAc;37%,28%)和海马(HIP;53%,30%)中的D(2)结合,但喹硫平没有。奥氮平和利培酮,而不是喹硫平,在CPu(61%,37%)、NAc(65%,32%)和HIP(61%,37%)中产生了更大的D(4)受体上调。所有区域的D(1)样和D(3)受体均未因任何治疗而改变,表明它们在介导这些抗精神病药物作用中的作用很小。这些发现支持这样一种假设,即奥氮平和利培酮的抗精神病作用部分由MPC、NAc或HIP中的D(2)受体介导,也可能由CPu、NAc和HIP的D(4)受体介介导,但不在大脑皮层。奥氮平和利培酮在CPu中选择性上调D(2)受体可能反映了它们诱导一些锥体外系效应的能力。喹硫平不能改变DA受体,这表明非多巴胺能机制有助于其抗精神病作用。[2] 肥胖和相关代谢紊乱是一些抗精神病药物(AP)的重要副作用。目前可用的AP诱导大鼠体重增加(BWG)的动物模型是基于舒必利(SUL)的给药。然而,这种模式有重要的局限性。例如,SUL是一种纯多巴胺拮抗剂,而目前临床使用的大多数AP与参与食物摄入(FI)和代谢调节的多种神经递质受体相互作用。因此,我们评估了利培酮/Risperidone(RIS,0.125、0.25或0.5mg/kg,持续16天)对雄性和雌性大鼠BWG和FI的影响。还对雌性大鼠进行了12天内RIS(0.5 mg/kg)、SUL(20 mg/kg)和赋形剂(VEH)的比较。在雄性大鼠中,RIS对BWG、FI、葡萄糖耐量或瘦素水平没有显著影响,尽管催乳素和皮质酮显著升高。在女性中,两种AP都显著增加了BWG和FI,但SUL的效果更强。BWG与体脂增加显著相关。血清瘦素水平仅在SUL治疗的大鼠中升高。SUL组的葡萄糖曲线下面积(AUGC)明显较低,但所有治疗组的胰岛素曲线下面积相似。胰岛素曲线下面积(AUIC)和BWG仅在RIS组呈正相关。两种AP均显著增加催乳素和皮质酮。RIS显著提高了血清雌二醇水平,但SUL没有,但两组的孕酮水平相似。在RIS给药期间观察到的BWG和AUIC之间的正相关表明,该药物可能代表了人类AP给药的更好模型。通过测试其他剂量、给药途径和饮食类型,可以改善RIS诱导的大鼠肥胖动物模型。[5] 1.利培酮/Risperidone是一种非典型抗精神病药物,具有5-羟色胺5-HT2受体拮抗作用和较轻的多巴胺D2受体拮抗作用。2.体重过度增加是抗精神病药物的副作用之一。利培酮治疗比氟哌啶醇等传统抗精神病药物治疗导致患者体重增加更多。因此,本研究旨在通过检测瘦素(一种在白色脂肪组织(WAT)中产生的食欲调节激素)和解偶联蛋白(UCP)-1(一种促进棕色脂肪组织(BAT)能量消耗的物质)的表达,来解决利培酮诱导的大鼠体重变化的病因。3.8周龄雄性大鼠皮下注射利培酮(0.005、0.05或0.5mg/kg),每天两次,持续21天。每天监测体重和食物摄入量。在第21天,将大鼠斩首,并测量其血清瘦素和催乳素浓度。使用实时聚合酶链式反应扩增定量WAT和BAT中瘦素、Ucp1和β3肾上腺素受体(β3-AR)基因的表达水平。4.大鼠注射0.005 mg/kg利培酮可增加食物摄入量和体重增加率,并增加WAT中瘦素基因的表达。注射0.05mg/kg利培酮可增加WAT的食物摄入量和瘦素基因表达,但体重增加率不受影响。注射0.5mg/kg利培酮可减少体重增加,并增强BAT和血清催乳素浓度中的Ucp1基因表达。注射0.5mg/kg利培酮对WAT和BAT的血清瘦素浓度和β3-AR基因表达没有影响。5.尽管在注射利培酮的大鼠中观察到的食物摄入量的变化既不是由血清瘦素也不是催乳素浓度合理化的,但注射0.5mg/kg后体重增加率的降低在一定程度上可以解释为能量消耗的增加,正如BAT中UCP-1 mRNA表达水平的显著增加所揭示的那样。瘦素在利培酮诱导的体重增加变化中的作用仍有待澄清[6]。 |
| 细胞实验 |
细胞活力[3]
通过3-[4,5-二甲基噻唑-2-基]-2,5-二苯基溴化四唑(MTT)还原产物的比色测量来确定细胞存活率。在用或不用氟哌啶醇和利培酮/Risperidone处理后,从96孔板中取出原始培养基,在含有0.5mg/mL MTT的无酚红最低必需培养基的存在下,在37°C下孵育细胞2小时。然后向每个孔中加入100 mL的酸-异丙醇(0.04 mol/L盐酸),并将平板在37°C下孵育过夜,以溶解孔中形成的甲氮。MTT在活细胞的线粒体中被还原为甲氮。通过平板读数器在570nm波长下测量MTT减少。 亚硝酸盐产量评估[3] NO2-是一种稳定的最终产物,广泛用作培养细胞产生NO的指标,其积累是通过格里斯反应进行测定的。将6-3个细胞以每200μl/孔1×105/孔的比例铺在96孔组织培养板上,然后在有或没有氟哌啶醇或Risperidone/利培酮的情况下预孵育12小时,然后在37°C下有或没有50 U/ml IFN-γ的情况下孵育。48小时后,将无细胞上清液与等量的Griess试剂混合。样品在室温下孵育15分钟,随后使用平板读数器在540nm处读取吸光度。 Western印迹法检测诱导型一氧化氮合酶(iNOS)[3] 将6-3个小胶质细胞以每孔1.8×106个细胞的密度铺在35 mm的组织培养皿上,然后在有或没有氟哌啶醇或Risperidone/利培酮的情况下预孵育12小时,然后在37°C下在有或无50 U/ml IFN-γ的情况下孵育12 h。之后,用PBS(pH 7.4)洗涤细胞,并用含十二烷基硫酸钠(SDS)的样品缓冲液裂解。蛋白质在7.5%的SDS-聚丙烯酰胺凝胶中分离,并转移到硝化纤维膜上。将膜与5%脱脂奶粉一起孵育,以阻断非特异性结合。随后,将膜与iNOS抗体和β-actin抗体一起孵育。采用增强化学发光系统检测iNOS和β-actin的表达。用光密度扫描仪对条带强度进行定量。实验分别进行了三次。 细胞因子释放评估[3] 将6-3个细胞以每200μl/孔1×105/孔的比例铺在96孔组织培养板上,然后在有或没有氟哌啶醇或Risperidone/利培酮的情况下预孵育12小时,然后在37°C下有或没有50 U/ml IFN-γ的情况下孵育。48小时后,检测收集的培养基中细胞因子(IL-1β、IL-6和TNF-α)的积累。基于定量“夹心”酶免疫吸附技术,使用小鼠IL-1β、IL-6和TNF-α酶联免疫吸附试验(ELISA)试剂盒测量释放到培养基中的细胞因子。根据制造商的方案进行了分析。该测定的灵敏度为4pg/ml。 细胞内Rh123和DOX积累研究[4] 测量P-gp底物Rh123和DOX的细胞内积累,以评估LLC-PK1/MDR1和Caco-2细胞中的P-gp活性,而LLC-PK1被列为阴性对照(van der Sandt等人,2000)。达到融合后,细胞在37°C下预孵育30 用运输缓冲液(无血清DMEM,含25 mM N-2-羟基哌嗪-N′-2-乙磺酸,pH 7.4)。加入载体对照(0.5%二甲亚砜(DMSO))、特定浓度的RSP/Risperidone/利培酮、PALI或PSC833,然后加入5 μM的Rh123或10 添加μM的DOX,再添加60 最小孵化时间。孵育后,丢弃溶液,用冰冷的DPBS洗涤细胞三次,并用1%Triton X-100溶解。通过高效液相色谱法(HPLC)测定Rh123和DOX的荧光。通过构建Rh123和DOX标准曲线,根据荧光值确定浓度。每个样品中Rh123或DOX的量用Lowry测定法测定的蛋白质含量进行标准化。 Rh123运输研究[4] 当RBMEC或Caco-2细胞达到融合时,通过TEER值和荧光素(一种公认的细胞旁转运标志物)的转运速率来检查单层的完整性(van Bree等人,1988)。用DPBS冲洗合格的单层两次,并在37°C下用运输缓冲液预孵育30 min.总之,在单层的两侧分别加载0.5%DMSO、RSP/利培酮或PSC833,然后在基底外侧加入Rh123(5μM)进行基底外侧到顶端(B-A)转运研究,或在顶端侧加入Rh123进行顶端到基底外侧(A-B)转运研究。在指定时间,150 从接收器室中取出μl样品,每次取样后立即更换相同体积的接收器室溶液。通过HPLC测定Rh123的浓度。表观渗透系数Papp(cm/s)根据以下方程式计算. |
| 动物实验 |
A total of 211 Long-Evans rats are utilized, comprising 56 females and 155 males. Three groups of approximately equal numbers of rats are injected with either 1.0 mg/kg of risperidone, 3.0 mg/kg of risperidone, or the vehicle used to administer the Risperidone solution as a control within each study. Twenty-six male rats (n = 9 in the vehicle and 3.0 mg/kg Risperidone groups; n = 8 in the 1.0 mg/kg Risperidone group) are used in the first experiment. They are tested for locomotor activity for 20 minutes every day starting on postnatal day 49 and continuing every day until postnatal day 53. The long-term effects of early-life Risperidone treatment on locomotion were examined in a follow-up study. In a third experiment, the effects of sex on early-life Risperidone's locomotor effects in young adult rats are investigated. Sixty male (n = 20 per treatment group) and fifty-six female (n = 19 rats in the vehicle and 3.0 mg/kg dose group, n = 18 in the 1.0 mg/kg dose group) rats are treated in this experiment. In a fourth experiment, rats given risperidone early in life were evaluated for reversal learning during adulthood. Treatment is given to 42 male rats (n=14 per treatment group).
Drug treatment [5] Racemic SUL and RisperidoneRIS were dissolved in 0.1 N HCl and tartaric acid respectively, and pH was adjusted to 7.0. Drugs were administered subcutaneously in a volume of 0.1 cc/100 g. Blood sampling and oral glucose tolerance test [5] General anesthesia was achieved by intramuscular administration of a solution made of Ketamine HCl (50 mg/ml), Xylazine HCl (5 mg/kg) and Acepromazine (1 mg/kg). The total volume was 1 ml/kg of BW. A catheter was placed in the tail dorsal artery and was filled with heparinized (2 U/ml) physiological saline at 12:00. The oral glucose tolerance test (glucose, 1 g/kg per gavage) was conducted in a counterbalanced order 36 h after surgery. Blood samples (0.1 cc) were removed before and after the glucose administration (at 0, 30, 60, 90 and 120 min; total 0.6 cc of blood) while the animal was gently placed in a plastic rodent restrainer. The catheter was immediately withdrawn at the end of the glucose tolerance test, and 2 days later the rats were decapitated after 6 hours of fasting. Trunk blood was collected for hormonal determination. For this purpose all animals were decapitated in a counterbalanced sequence between 17:00 and 19:00 h. In order to minimize the effects of stress on hormone levels, less than 1 minute elapsed between handling the animals and decapitation. Bodies were processed for body composition analysis (see below). Experiment 1: Effects of Risperidone on BW and FI in female or male rats [5] For each gender, 32 animals were randomly assigned to 4 groups of 8 subjects each, which received one of the following treatments: vehicle (0.1 cc/kg), Risperidone/RIS 0.125, 0.25 or 0.5 mg/kg for 16 days. These doses of RIS are known to induce little impairment in motor behavior in rats. Measurement of BWG and FI was conducted as stated above. Since no additional studies were carried out in males, an oral glucose tolerance test was conducted in the rats treated with VEH and RIS 0.5 mg/kg at day 14. Serum glucose and insulin levels were assessed in all blood samples. Leptin, corticosterone and prolactin levels were measured in blood samples collected immediately after decapitation of the animals at the end of the experiment. Experiment 2: Comparison between the effects of Risperidone and sulpiride on BW, FI, glucose tolerance, vaginal cycle, hormones and glucose tolerance in female rats [5] Twenty-nine female rats were divided into 3 groups that received one of the following treatments for 12 days: 0.9% NaCl, 0.1 cc/kg (n=9), RIS 0.5 mg/kg (n=11) or SUL 20 mg/kg (n=9). This SUL dose has been shown to induce the maximal BWG after prolonged treatment. The intra-arterial catheter was placed on day 9 after onset of drug treatment, and was withdrawn when the glucose test was completed. On day 10 the animals were fasted for 6 hours and then the oral glucose tolerance test was conducted as described in the blood sampling section. Insulin and glucose were assessed in all blood samples. Leptin, corticosterone, prolactin, progesterone and estradiol concentrations were assessed in the basal samples obtained by decapitation on day 12. Risperidone was used. Eight-week-old male Sprague-Dawley rats, weighing 285–305 g, were housed individually, maintained on a 12 h light/dark cycle (lights on at 06.00 h) and allowed free access to standard rodent food (CE-2; 14.4 kJ/g, consisting of 12% fat, 29% protein and 59% carbohydrate) and water. Room temperature was maintained at 23 ± 1°C. After habituation for 3 days, rats were divided into four experimental groups. Three groups were injected twice daily (09.00 and 18.00 h) for 21 days by subcutaneous (s.c.) injection into the neck region with Risperidone at concentrations of 0.005, 0.05 or 0.5 mg/kg (n = 5, 6 and 5, respectively), with rats in their home chambers. Another 10 rats received s.c. injection into the neck region of vehicle (0.3% tartaric acid) twice daily at 09.00 and 18.00 h for 21 days. Bodyweight and food intake were recorded daily to the nearest 1.0 g just before the injection at 09.00 h. On day 21, 1 h after the 18.00 h injection, rats were decapitated. Blood samples were obtained from the trunk vessels and the sera were kept at −80°C until hormone assays could be performed. The epididymal WAT and interscapular BAT were rapidly removed and stored at −80°C prior to RNA extraction [6]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution. Risperidone is extensively metabolized in the liver. In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives are prolonged compared to young healthy subjects. The volume of distribution of risperidone is approximately 1 to 2 L/kg. Risperidone is cleared by the kidneys. Clearance is decreased in the elderly and those with a creatinine clearance (ClCr) between 15-59 mL/min, in whom clearance is decreased by approximately 60%. Risperidone is well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution. Risperidone is rapidly distributed. The volume of distribution is 1-2 L/kg. In plasma, risperidone is bound to albumin and a1-acid glycoprotein. The plasma protein binding of risperidone is 90%, and that of its major metabolite, 9-hydroxyrisperidone, is 77%. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites. High therapeutic concentrations of sulfamethazine (100 ug/mL), warfarin (10 ug/mL), and carbamazepine (10 ug/mL) caused only a slight increase in the free fraction of risperidone at 10 ng/mL and 9-hydroxyrisperidone at 50 ng/mL, changes of unknown clinical significance. Plasma concentrations of risperidone, its major metabolite, 9-hydroxyrisperidone, and risperidone plus 9-hydroxyrisperidone are dose proportional over the dosing range of 1 to 16 mg daily (0.5 to 8 mg twice daily). Following oral administration of solution or tablet, mean peak plasma concentrations of risperidone occurred at about 1 hour. Peak concentrations of 9-hydroxyrisperidone occurred at about 3 hours in extensive metabolizers, and 17 hours in poor metabolizers. Steady-state concentrations of risperidone are reached in 1 day in extensive metabolizers and would be expected to reach steady-state in about 5 days in poor metabolizers. Steady-state concentrations of 9-hydroxyrisperidone are reached in 5-6 days (measured in extensive metabolizers). Risperidone and 9-hydroxyrisperidone are present in human breast milk. For more Absorption, Distribution and Excretion (Complete) data for RISPERIDONE (6 total), please visit the HSDB record page. Metabolism / Metabolites Extensively metabolized by hepatic cytochrome P450 2D6 isozyme to 9-hydroxyrisperidone (i.e. [paliperidone]), which has approximately the same receptor binding affinity as risperidone. Hydroxylation is dependent on debrisoquine 4-hydroxylase and metabolism is sensitive to genetic polymorphisms in debrisoquine 4-hydroxylase. Risperidone also undergoes N-dealkylation to a lesser extent. Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme, CYP 2D6. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone. CYP 2D6, also called debrisoquin hydroxylase, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP 2D6 is subject to genetic polymorphism (about 6%-8% of Caucasians, and a very low percentage of Asians, have little or no activity and are "poor metabolizers") and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP 2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP 2D6 metabolizers convert it much more slowly. Although extensive metabolizers have lower risperidone and higher 9-hydroxyrisperidone concentrations than poor metabolizers, the pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, are similar in extensive and poor metabolizers. Risperidone has known human metabolites that include 9-Hydroxy-risperidone, Paliperidone, 3-[2-[4-(6-fluoro-2-hydroxy-1,2-benzoxazol-2-ium-3-yl)piperidin-1-yl]ethyl]-2,9-dimethyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one, 3-ethyl-2,9-dimethyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one, and 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole. Extensively metabolized by hepatic cytochrome P450 2D6 isozyme to 9-hydroxyrisperidone, which has approximately the same receptor binding affinity as risperidone. Hydroxylation is dependent on debrisoquine 4-hydroxylase and metabolism is sensitive to genetic polymorphisms in debrisoquine 4-hydroxylase. Risperidone also undergoes N-dealkylation to a lesser extent. Route of Elimination: Risperidone is extensively metabolized in the liver.In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives were prolonged compared to young healthy subjects. Half Life: 20-24 hours Biological Half-Life 3 hours in extensive metabolizers Up to 20 hours in poor metabolizers The apparent half-life of risperidone plus 9-hydroxyrisperidone following Risperdal Consta administration is 3 to 6 days, and is associated with a monoexponential decline in plasma concentrations. This half-life of 3-6 days is related to the erosion of the microspheres and subsequent absorption of risperidone. The apparent half-life of risperidone was 3 hours (CV=30%) in extensive metabolizers and 20 hours (CV=40%) in poor metabolizers. The apparent half-life of 9-hydroxyrisperidone was about 21 hours (CV=20%) in extensive metabolizers and 30 hours (CV=25%) in poor metabolizers. The pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, were similar in extensive and poor metabolizers, with an overall mean elimination half-life of about 20 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Blockade of dopaminergic D2 receptors in the limbic system alleviates positive symptoms of schizophrenia such as hallucinations, delusions, and erratic behavior and speech. Blockade of serotonergic 5-HT2 receptors in the mesocortical tract, causes an excess of dopamine and an increase in dopamine transmission, resulting in an increase in dopamine transmission and an elimination of core negative symptoms. Dopamine receptors in the nigrostriatal pathway are not affected by risperidone and extrapyramidal effects are avoided. Like other 5-HT2 antagonists, risperidone also binds at alpha(1)-adrenergic receptors and, to a lesser extent, at histamine H1 and alpha(2)-adrenergic receptors. Toxicity Data LD50=82.1mg/kg (orally in mice). Interactions Given the primary CNS effects of risperidone, caution should be used when Risperdal is taken in combination with other centrally-acting drugs and alcohol. Risperdal may antagonize the effects of levodopa and dopamine agonists. When Risperdal is co-administered with enzyme inducers (e.g., carbamazepine), the dose of Risperdal should be increased up to double the patient's usual dose. It may be necessary to decrease the Risperdal dose when enzyme inducers such as carbamazepine are discontinued [see Drug Interactions (7.1)]. Similar effect may be expected with co-administration of Risperdal with other enzyme inducers (e.g., phenytoin, rifampin, and phenobarbital). Chronic administration of clozapine with Risperdal may decrease the clearance of risperidone. For more Interactions (Complete) data for RISPERIDONE (10 total), please visit the HSDB record page. Hepatotoxicity Liver test abnormalities may occur in up to 30% of patients on long term therapy with risperidone, usually arising within the first 8 weeks of treatment. The ALT elevations are usually mild, transient and may resolve even with continuation of medication. Instances of more marked ALT and alkaline phosphatase elevations, with or without symptoms and with or without jaundice, have also been reported. The onset of injury typically occurs within a few days of starting risperidone and resolves rapidly with stopping. Instances of acute liver injury with jaundice arising several months and even years after starting risperidone have also been reported. The pattern of serum enzyme elevations is typically cholestatic, but cases with hepatocellular and mixed patterns have also been described. Immunoallergic manifestations (rash, fever, eosinophilia) are rare; a case of autoimmune hepatitis apparently triggered by risperidone therapy has been published, but most cases do not have autoimmune features. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal risperidone doses of up to 6 mg daily produce low levels in milk. Sedation, failure to thrive, jitteriness, tremors, abnormal muscle movements and respiratory depression have been reported in infants exposed to risperidone in milk. Because there is little published experience with risperidone during breastfeeding and little long-term follow-up data, other agents may be preferred, especially while nursing a newborn or preterm infant. Systematic reviews of second-generation antipsychotics concluded that risperidone seemed to be a second-line agent during breastfeeding because of the limited data available and higher excretion into milk relative to other agents. A safety scoring system finds risperidone to be possible to use cautiously during breastfeeding. Monitor the infant for drowsiness, weight gain, tremors, respiratory rate, abnormal muscle movements, and developmental milestones, especially if other antipsychotics are used concurrently. ◉ Effects in Breastfed Infants One woman took risperidone 4 mg daily during breastfeeding. Her infant showed no developmental abnormalities on examinations up to 9 months of age. Another mother took risperidone 6 mg daily during breastfeeding. Her infant showed no developmental abnormalities on examinations up to 12 months of age. Two women taking risperidone 4 mg and 1.5 mg daily breastfed their infants of 3.3 months and 6 weeks of age, respectively, were achieving normal developmental milestones and had no adverse effects reported. A 1 week postpartum woman was started on risperidone 2 mg daily and increased after 10 days to a dosage of 3 mg daily. She breastfed her infant 6 times daily. The infant was observed for 5 weeks of inpatient therapy and judged normal by a pediatric neurologist. No sedation or other adverse effects were observed in the infant. After 3 months of treatment with risperidone, the mother and infant were judged to be well. An infant had been exclusively breastfed for 3 months during maternal therapy with risperidone 1 mg daily. A pediatric examination found the infant to have no neurological or physical abnormalities, and appeared to interact appropriately. In a telephone follow-up study, 124 mothers who took a benzodiazepine while nursing reported whether their infants had any signs of sedation. One mother who was taking 0.75 mg of risperidone daily, flurazepam 15 mg daily, clonazepam 0.25 mg twice daily, and 1 mg of bupropion daily reported sedation in her breastfed infant. A woman diagnosed with schizophrenia was taking risperidone 1.5 mg daily during late pregnancy and postpartum while nursing (extent not stated) her full-term infant. At 2 weeks postpartum, haloperidol 0.8 mg daily was added because of a recurrence of symptoms. At these dosages, no adverse effects were seen in the infant. However, because of recurring symptoms, the dosage of haloperidol was increased to 1.5 mg daily. Three days later, the infant had excessive sedation, poor feeding, and slowing in motor movements. Pediatric assessment found no medical reason for these effects. Breastfeeding was discontinued and the infant's symptoms resolved completely in 5 days. The infant's symptoms were probably caused by the drug combination. A prospective cohort study of infants breastfed by mothers in an inpatient mother-baby psychiatric unit in India followed 7 infants who were exposed to risperidone in breastmilk; most received partial supplementation. One infant whose mother was taking risperidone 4 mg and lorazepam 2 mg developed sedation that resolved when lorazepam was discontinued. One infant whose mother received risperidone 4 mg daily, trihexyphenidyl 2 mg daily, and electroconvulsive therapy developed constipation. Infants were followed for 1 to 3 months after discharge. One infant had delayed weight development, one infant had delay in height, one infant mental delay, and a fourth infant had motor and mental delay. A woman with bipolar disorder was maintained on oral risperidone 2 mg at bedtime, long-acting injectable risperidone 50 mg intramuscular every 2 weeks, oral citalopram 20 mg daily, and oral benztropine 0.5 mg daily. She became pregnant and maintained the same regimen. Her infant was born at 35 weeks gestational age and was breastfed (extent and duration not stated). At 16 months of age, the infant was doing well and met his developmental milestones. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who were not treated with a second-generation antipsychotic (n = 818). Of the patients who were taking a second-generation antipsychotic drug, 60.4% were on more than one psychotropic. A review of the pediatric medical records, no adverse effects were noted among infants exposed or not exposed to second-generation antipsychotic monotherapy or to polytherapy. The number of women taking risperidone was not reported. A preterm infant weighing 2.75 kg was born at 35 weeks gestation. The infant received bag and mask ventilation for 2 min and was kept on oxygen for the first 18 hours of life due to respiratory distress. The baby began breastfeeding on day 2 of life. On day 12, the mother was started on risperidone 1 mg daily for psychotic episodes. On day 13, the infant developed a respiratory rate of 16/min and no retractions and was placed on CPAP for 12 hours, with gradual weaning thereafter and was placed on formula. On day 15, the mother began breastfeeding again and the respiratory depression recurred. Feeding was changed to breastmilk expressed prior to the daily dose of risperidone and formula for 6 hours after each dose followed by direct breastfeeding. Over the next 2 days no further episodes of respiratory depression occurred. The baby was discharged on day 24, with advice to continue the same feeding pattern. Respiratory depression was probably caused by risperidone in milk. A woman diagnosed with undifferentiated schizophrenia took risperidone 4 to 5 mg and trihexyphenidyl 2 mg daily throughout 5 pregnancies. She breastfed each infant for 20 to 24 months. No adverse developmental consequences were noted in any of the children. At the time of publication, the oldest three children, aged 26, 23 and 22 years, had completed their education and were employed, while the youngest two were 15 and 19 years old and were doing well academically in their education. ◉ Effects on Lactation and Breastmilk Risperidone has caused elevated prolactin serum levels, gynecomastia, and galactorrhea in patients taking the drug. In one case, euprolactinemic gynecomastia and galactorrhea occurred in a 19-year-old man who was also taking fluvoxamine. A meta-analysis of 3 studies found that the risk of gynecomastia with risperidone is 4.3 times greater than that of quetiapine. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who primarily had diagnoses of major depressive disorder and anxiety disorders, most often treated with SSRI or SNRI antidepressants, but not with a second-generation antipsychotic (n = 818). Among women on a second-generation antipsychotic, 60.4% were on more than one psychotropic compared with 24.4% among women in the control group. Of the women on a second-generation antipsychotic, 59.3% reported “ever breastfeeding” compared to 88.2% of women in the control group. At 3 months postpartum, 23% of women on a second-generation antipsychotic were exclusively breastfeeding compared to 47% of women in the control group. The number of women taking risperidone was not reported. ◈ What is risperidone? Risperidone is a medication that has been used to treat mental health conditions such as schizophrenia, bipolar disorder, and depression. It can be taken by mouth or given as an injection. Risperidone belongs to a group of medications called atypical or second-generation antipsychotics. Brand names for risperidone include Risperdal®, Risperdal Consta®, and Perseris®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take risperidone. Can it make it harder for me to get pregnant? In some people, risperidone may raise the levels of a hormone called prolactin. High levels of prolactin can stop ovulation (part of the menstrual cycle when an ovary releases an egg). This would make it harder to get pregnant. Your healthcare provider can test your levels of prolactin if there is concern. ◈ Does taking risperidone increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Based on the studies reviewed, risperidone is not expected to increase the chance of miscarriage. ◈ Does taking risperidone increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Based on the studies reviewed, risperidone is not expected to increase the chance of birth defects above the background risk. ◈ Does taking risperidone in pregnancy increase the chance of other pregnancy-related problems? Based on the studies reviewed, risperidone may cause low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth).Risperidone may cause weight gain and problems with blood sugar in a person who is pregnant. This may increase the chance of developing gestational diabetes. More information about gestational diabetes can be found in our fact sheet https://mothertobaby.org/fact-sheets/diabetes-pregnancy/. ◈ I need to take risperidone throughout my entire pregnancy. Will it cause withdrawal symptoms in my baby after birth? The use of some medications during pregnancy may cause temporary symptoms in newborns soon after birth. These symptoms are sometimes referred to as withdrawal. It is unknown if taking risperidone alone could increase the chance of withdrawal symptoms in a newborn. Similar medications have been associated with a chance for withdrawal, so babies exposed to risperidone near the time of delivery should be watched for stiff or floppy muscles, drowsiness, agitation, tremors, trouble breathing, and problems with feeding. In most cases, symptoms would be expected to go away in a few days without any long-term health effects. It is important that your healthcare providers know you are taking risperidone so that if symptoms occur your baby can get the care that is best for them. ◈ Does taking risperidone in pregnancy affect future behavior or learning for the child? Studies have not been done to see if risperidone can cause behavior or learning issues for the child. ◈ Breastfeeding while taking risperidone: Information on the use of risperidone during breastfeeding is limited. When taken in doses of up to 6 mg a day risperidone was found in breastmilk in small amounts. Side effects were not reported in a small number of breastfed infants who were exposed to risperidone only (in doses of up to 6 mg a day). If you take risperidone and other medications, there may be a higher chance for side effects in the baby. If you suspect the baby has any symptoms (sleepiness, poor feeding, crankiness, or unusual movements) contact the child’s healthcare provider.The product label for risperidone recommends that people who are breastfeeding not use this medication. But the benefit of using risperidone may outweigh the possible risks. Your healthcare providers can talk with you about using risperidone and what treatment is best for you. Be sure to talk to your healthcare provider about all of your breastfeeding questions. ◈ If a male takes risperidone, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? Using risperidone may raise a person’s levels of the hormone prolactin, which may affect fertility. Studies have not been done to see if risperidone could increase the chance of birth defects above the background risks. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antipsychotic Agents; Dopamine Antagonists; Serotonin Antagonists Risperdal (risperidone) is indicated for the treatment of schizophrenia. Efficacy was established in 4 short-term trials in adults, 2 short-term trials in adolescents (ages 13 to 17 years), and one long-term maintenance trial in adults /Included in US product label/ Risperdal adjunctive therapy with lithium or valproate is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in one short-term trial in adults. /Included in US product label/ Risperdal is indicated for the treatment of irritability associated with autistic disorder, including symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods. Efficacy was established in 3 short-term trials in children and adolescents (ages 5 to 17 years). /Included in US product label/ Risperdal is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in 2 short-term trials in adults and one short-term trial in children and adolescents (ages 10 to 17 years). /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Risperdal (risperidone) is not approved for the treatment of patients with dementia-related psychosis. Like other antipsychotic agents (e.g., phenothiazines), risperidone has been associated with tardive dyskinesias. Although it has been suggested that atypical antipsychotics appear to have a lower risk of tardive dyskinesia, whether antipsychotic drugs differ in their potential to cause tardive dyskinesia is as yet unknown. In one open-label study, an annual incidence of tardive dyskinesia of 0.3% was reported in patients with schizophrenia who received approximately 8-9 mg of oral risperidone daily for at least 1 year. The prevalence of this syndrome appears to be highest among geriatric patients (particularly females). The risk of developing tardive dyskinesia and the likelihood that it will become irreversible also appear to increase with the duration of therapy and cumulative dose of antipsychotic agents administered; however, the syndrome may occur, although much less frequently, after relatively short periods of treatment with low dosages. Neuroleptic malignant syndrome (NMS), a potentially fatal symptom complex, has been reported in patients receiving antipsychotic agents. NMS requires immediate discontinuance of the drug and intensive symptomatic and supportive care. Dose-related somnolence was a commonly reported adverse effect associated with risperidone treatment. Approximately 8% of adult patients with schizophrenia receiving 16 mg of oral risperidone daily and 1% of patients receiving placebo reported somnolence in studies utilizing direct questioning or a checklist to detect adverse events, respectively. For more Drug Warnings (Complete) data for RISPERIDONE (41 total), please visit the HSDB record page. Pharmacodynamics The primary action of risperidone is to decrease dopaminergic and serotonergic pathway activity in the brain, therefore decreasing symptoms of schizophrenia and mood disorders. Risperidone has a high binding affinity for serotonergic 5-HT2A receptors when compared to dopaminergic D2 receptors in the brain. Risperidone binds to D2 receptors with a lower affinity than first-generation antipsychotic drugs, which bind with very high affinity. A reduction in extrapyramidal symptoms with risperidone, when compared to its predecessors, is likely a result of its moderate affinity for dopaminergic D2 receptors. Antipsychotics have been regarded to effect mainly neurons or neural networks including the synapse network for a long period of time. However, the present study has demonstrated that antipsychotics, especially risperidone, have an anti-inflammatory effect via the inhibition of microglial activation. Antipsychotics may therefore have a potentially useful therapeutic effect on schizophrenic patients by reducing microglial inflammatory reactions, which may inhibit the process of neurogenesis and oligodendrogenesis. These results might shed some new light on the therapeutic strategies for the treatment of schizophrenia. Agents that can inhibit microglial activation may also be useful for the treatment of schizophrenia. For further studies, the molecular mechanism of the inhibitory effect of Risperidone on microglial activation should be clarified in detail while in vivo studies to confirm the present results should also be performed.[3] The mechanistic basis of P-gp transport and inhibition has been intensively studied for many years. Several hypotheses have been developed in an effort to explain the molecular mechanism of interaction of P-gp and its substrates or inhibitors. However, owing to multiple drug binding sites on P-gp, the development of a universally accepted model for reconciling the data from various laboratories remains a challenge. It has been demonstrated that there is a minimum of four drug binding sites on P-gp. These sites can be divided into two categories: transport sites, at which translocation of drug across the cellular membrane can occur, and regulatory sites, which modify P-gp function (Martin et al, 2000). In addition, some agents can inhibit P-gp activity by decreasing intracellular ATP supply and inhibiting P-gp ATPase activity (Batrakova et al, 2001). Considering the fact that both Risperidone/RSP and PALI appear to be P-gp substrates as well as inhibitors, competition with other substrates for binding to P-gp is a possible mechanism of their P-gp inhibition. However, other mechanisms cannot be excluded and further studies are needed to elucidate the molecular basis of RSP and PALI interactions with P-gp.[4] A final answer regarding an explanation for the alteration in food intake of Risperidone-injected rats was not obtained in the present study from the evidence involving dopamine and/or 5-HT receptor antagonism. However, the reduction in bodyweight gain in risperidone (0.5 mg/kg)-injected rats may be explained, in part, by increased energy expenditure, as revealed by the remarkable increase in UCP-1 mRNA expression in BAT. In humans, BAT is found in the newborn, but it becomes less abundant in adults, in whom the role of BAT is minor.73 This may partially explain the difference in the effect of risperidone on bodyweight gain between rodents and humans. In the present study, we have demonstrated, for the first time, that risperidone injection in rats induced leptin mRNA expression in WAT and UCP-1 mRNA expression in BAT. However, we were not able to provide a rational explanation for these elevated expressions. Thus, our next task is to define the basis for this risperidone-induced increase in mRNA expression levels of these genes in the adipose tissues.[6] |
| 分子式 |
C23H27FN4O2
|
|
|---|---|---|
| 分子量 |
410.48
|
|
| 精确质量 |
410.211
|
|
| 元素分析 |
C, 67.30; H, 6.63; F, 4.63; N, 13.65; O, 7.80
|
|
| CAS号 |
106266-06-2
|
|
| 相关CAS号 |
Risperidone-d4; 1020719-76-9; Risperidone hydrochloride; 666179-74-4; Risperidone mesylate; 666179-96-0
|
|
| PubChem CID |
5073
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
572.4±60.0 °C at 760 mmHg
|
|
| 熔点 |
170°C
|
|
| 闪点 |
300.0±32.9 °C
|
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
|
| 折射率 |
1.677
|
|
| LogP |
2.88
|
|
| tPSA |
64.16
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
731
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
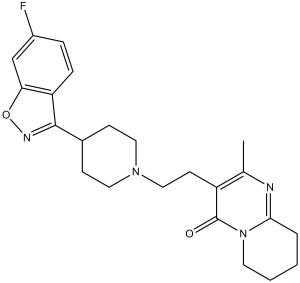
FC1C([H])=C([H])C2=C(C=1[H])ON=C2C1([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C2=C(C([H])([H])[H])N=C3C([H])([H])C([H])([H])C([H])([H])C([H])([H])N3C2=O)C([H])([H])C1([H])[H]
|
|
| InChi Key |
RAPZEAPATHNIPO-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H
|
|
| 化学名 |
3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (2.44 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (2.44 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (2.44 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4362 mL | 12.1809 mL | 24.3617 mL | |
| 5 mM | 0.4872 mL | 2.4362 mL | 4.8723 mL | |
| 10 mM | 0.2436 mL | 1.2181 mL | 2.4362 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
CLOZAPINE Response in Biotype-1
CTID: NCT04580134
Phase: Phase 4 Status: Recruiting
Date: 2024-08-28
|
|---|