| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
5-HT2 Receptor ( Ki = 4.8 nM ); D2 Receptor ( Ki = 5.9 nM ); P-Glycoprotein
|
|---|---|
| 体外研究 (In Vitro) |
Risperidone 是一种血清素 5-HT2 受体阻滞剂、P-糖蛋白抑制剂和有效的多巴胺 D2 受体拮抗剂,对 5-HT2A 和多巴胺 D2 受体的 Kis 分别为 4.8 和 5.9 nM。利培酮剂量依赖性地抑制成熟DC中IL-12的释放,而利培酮剂量依赖性地增加IL-10的产生。高剂量的利培酮可以诱导成熟 DC 释放 TNF-α[3]。
主要与多巴胺D2受体或II型5-HT受体结合的抗精神病药物(APD)已被用于缓解精神分裂症的症状。几项研究报告称,APD也可以调节免疫反应。树突状细胞(DC)是免疫系统中主要的抗原呈递细胞。DC可以释放5-HT和多巴胺来调节T细胞的活化和分化。在这项研究中,我们使用单核细胞衍生的DC来研究典型APD(氟哌啶醇)和非典型APD(利培酮)对DC的体外药物作用。我们的研究表明,只有利培酮而不是氟哌啶醇影响成熟树突状细胞的细胞因子和趋化因子产生。利培酮增加了IL-10和MDC以及促炎细胞因子如IL-6、IL-8和TNF-α的产生,但降低了IP-10和IL-12的产生。此外,DC暴露于利培酮会导致T细胞产生较低的IFN-γ。结果表明,利培酮可以通过抑制强效Th1细胞因子和增加强效Th2细胞因子来调节树突状细胞的免疫功能。此外,利培酮处理的成熟树突状细胞产生TNF-α会诱导中性粒细胞死亡。[2] 利培酮(RSP)及其主要活性代谢产物9-羟基利培酮(帕利哌酮,PALI)是药物转运蛋白P-糖蛋白(P-gp)的底物。本研究的目的是研究RSP和PALI对P-糖蛋白介导的转运的体外影响。在LLC-PK1/MDR1细胞中检测罗丹明123(Rh123)和阿霉素(DOX)的细胞内积累,以评估RSP和PALI对P-gp的抑制作用。这两种化合物都以浓度依赖的方式显著增加了Rh123和DOX的细胞内积累。RSP抑制P-gp介导的Rh123和DOX转运的IC(50)值分别为63.26和15.78微M,而PALI的IC(50中)值>100微M,表明PALI是一种效力较弱的P-gp抑制剂。利用Caco-2和原代培养的大鼠脑微血管内皮细胞(RBMECs)研究RSP对作为P-gp底物的联合用药的肠道吸收和血脑屏障(BBB)转运的可能影响。RSP,1-50微M,通过抑制P-gp活性显著增强了Caco-2细胞中Rh123的细胞内积累,IC(50)值为5.87微M。暴露于10微M RSP后,Rh123在Caco-2和RBMECs单层上的表观渗透系数在顶端到基底外侧方向上分别增加到2.02和2.63倍,但在基底外侧到顶端方向上分别降低到0.37和0.21倍。这些数据表明,RSP和PALI在较小程度上有可能通过抑制P-gp介导的转运来影响药物动力学,从而影响联合用药的药效学。然而,目前还没有解决这个问题的人类数据。特别是,RSP可能通过抑制P-gp介导的PALI穿过BBB内皮细胞的流出来促进其脑浓度,从而与其自身的活性代谢产物PALI相互作用[3]。 |
| 体内研究 (In Vivo) |
在第一个实验中,发现利培酮治疗的大鼠的体重随着年龄的增长而略有下降,但显着降低。与第一个实验类似,在第二个运动实验中,三个治疗组之间也观察到了年龄依赖性的体重差异。在出生后第 35、38 和 41 天,用 3.0 mg/kg 剂量的利培酮治疗的大鼠体重低于媒介物治疗的大鼠。第三个运动实验涉及较大的混合性别窝,与使用的较小的单一性别窝相比在前两个实验中。正如前两个实验所指出的,在第三个实验中用利培酮治疗的大鼠以年龄依赖性方式体重增加较少[4]。
有人认为,联合阻断5-HT2和D2多巴胺受体在治疗精神分裂症方面可能优于单独使用D2多巴胺拮抗剂。利培酮在体外对5-HT2和D2多巴胺受体具有高亲和力,是根据这一假设开发的一种新型抗精神病药物。本研究的目的是检查利培酮是否确实在人体内诱导5-HT2和D2多巴胺受体占据。口服1mg利培酮后,通过正电子发射断层扫描(PET)检查了三名健康男性的中枢受体占有率。[11C]N-甲基螺吡喃酮([11C]NMSP)用作放射性配体,用于测定新皮层中5-HT2受体的占有率。平衡比分析和动力学三室分析均表明5-HT2受体占有率约为60%。[11C]雷氯必利用作放射性配体,用于测定纹状体中D2多巴胺受体的占有率,计算出的占有率约为50%。这是首次定量测定抗精神病药物在活体人脑中诱导的5-HT2受体占有率。结果表明,如临床使用所建议的那样,在每天4-10mg利培酮的剂量水平下,5-HT2受体占有率应该非常高。因此,利培酮是临床评估5-HT2和D2多巴胺受体联合阻断治疗精神分裂症益处的合适化合物。[1] 利培酮是一种经批准用于儿童的抗精神病药物,但人们对早期利培酮治疗的长期影响知之甚少。在动物中,在发育过程中长期服用利培酮会在治疗停止后立即增加前脑多巴胺受体的表达。进行了一系列实验,以确定早期服用利培酮是否会改变成年大鼠的运动活动,这是一种对多巴胺受体功能敏感的行为。在成年期,还测量了前脑多巴胺功能调节的另一种行为,即空间逆转学习。在每项研究中,Long-Evans大鼠在出生后第14至42天每天皮下注射赋形剂或2剂利培酮中的1剂(每天1.0和3.0mg/kg)。利培酮治疗的大鼠在发育过程中的体重增加略有但显著减少。在前两个实验中,早年服用利培酮与服用后1周至约9个月龄时运动活动的增加有关,与体重增加的变化无关。在另一项实验中,发现早期利培酮对雄性和雌性大鼠运动活动的增强作用。最后一项实验表明,成年大鼠在生命早期服用利培酮后,空间逆转学习不受影响。这些结果表明,成年期的运动活动会因早期利培酮治疗而永久改变。研究结果表明,在儿科人群中长期使用抗精神病药物(例如,治疗自闭症症状)可能会改变大脑发育,并改变成年期特定行为的神经设定点[4]。 |
| 细胞实验 |
细胞内Rh123和DOX积累研究[3]
测量P-gp底物Rh123和DOX的细胞内积累,以评估LLC-PK1/MDR1和Caco-2细胞中的P-gp活性,而LLC-PK1被列为阴性对照(van der Sandt等人,2000)。达到融合后,细胞在37°C下预孵育30 用运输缓冲液(无血清DMEM,含25 mM N-2-羟基哌嗪-N′-2-乙磺酸,pH 7.4)。加入载体对照(0.5%二甲亚砜(DMSO))、特定浓度的RSP/Risperidone/利培酮、PALI或PSC833,然后加入5 μM的Rh123或10 添加μM的DOX,再添加60 最小孵化时间。孵育后,丢弃溶液,用冰冷的DPBS洗涤细胞三次,并用1%Triton X-100溶解。通过高效液相色谱法(HPLC)测定Rh123和DOX的荧光。通过构建Rh123和DOX标准曲线,根据荧光值确定浓度。每个样品中Rh123或DOX的量用Lowry测定法测定的蛋白质含量进行标准化。 Rh123运输研究[3] 当RBMEC或Caco-2细胞达到融合时,通过TEER值和荧光素(一种公认的细胞旁转运标志物)的转运速率来检查单层的完整性(van Bree等人,1988)。用DPBS冲洗合格的单层两次,并在37°C下用运输缓冲液预孵育30 min.总之,在单层的两侧分别加载0.5%DMSO、RSP/利培酮或PSC833,然后在基底外侧加入Rh123(5μM)进行基底外侧到顶端(B-A)转运研究,或在顶端侧加入Rh123进行顶端到基底外侧(A-B)转运研究。在指定时间,150 从接收器室中取出μl样品,每次取样后立即更换相同体积的接收器室溶液。通过HPLC测定Rh123的浓度。表观渗透系数Papp(cm/s)根据以下方程式计算. |
| 动物实验 |
Rats: A total of 211 Long-Evans rats are utilized, comprising 56 females and 155 males. Three groups of approximately equal numbers of rats are injected with either 1.0 mg/kg of risperidone, 3.0 mg/kg of risperidone, or the vehicle used to administer the risperidone solution as a control within each study. Twenty-six male rats (n = 9 in the vehicle and 3.0 mg/kg Risperidone groups; n = 8 in the 1.0 mg/kg Risperidone group) are used in the first experiment. They are tested for locomotor activity for 20 minutes every day starting on postnatal day 49 and continuing every day until postnatal day 53. The long-term effects of early-life Risperidone treatment on locomotion were examined in a follow-up study. In a third experiment, the effects of sex on early-life Risperidone's locomotor effects in young adult rats are investigated. Sixty male (n = 20 per treatment group) and fifty-six female (n = 19 rats in the vehicle and 3.0 mg/kg dose group, n = 18 in the 1.0 mg/kg dose group) rats are treated in this experiment. In a fourth experiment, rats given risperidone early in life were evaluated for reversal learning during adulthood. Treatment is given to 42 male rats (n=14 per treatment group)[4].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution. Risperidone is extensively metabolized in the liver. In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives are prolonged compared to young healthy subjects. The volume of distribution of risperidone is approximately 1 to 2 L/kg. Risperidone is cleared by the kidneys. Clearance is decreased in the elderly and those with a creatinine clearance (ClCr) between 15-59 mL/min, in whom clearance is decreased by approximately 60%. Risperidone is well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution. Risperidone is rapidly distributed. The volume of distribution is 1-2 L/kg. In plasma, risperidone is bound to albumin and a1-acid glycoprotein. The plasma protein binding of risperidone is 90%, and that of its major metabolite, 9-hydroxyrisperidone, is 77%. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites. High therapeutic concentrations of sulfamethazine (100 ug/mL), warfarin (10 ug/mL), and carbamazepine (10 ug/mL) caused only a slight increase in the free fraction of risperidone at 10 ng/mL and 9-hydroxyrisperidone at 50 ng/mL, changes of unknown clinical significance. Plasma concentrations of risperidone, its major metabolite, 9-hydroxyrisperidone, and risperidone plus 9-hydroxyrisperidone are dose proportional over the dosing range of 1 to 16 mg daily (0.5 to 8 mg twice daily). Following oral administration of solution or tablet, mean peak plasma concentrations of risperidone occurred at about 1 hour. Peak concentrations of 9-hydroxyrisperidone occurred at about 3 hours in extensive metabolizers, and 17 hours in poor metabolizers. Steady-state concentrations of risperidone are reached in 1 day in extensive metabolizers and would be expected to reach steady-state in about 5 days in poor metabolizers. Steady-state concentrations of 9-hydroxyrisperidone are reached in 5-6 days (measured in extensive metabolizers). Risperidone and 9-hydroxyrisperidone are present in human breast milk. For more Absorption, Distribution and Excretion (Complete) data for RISPERIDONE (6 total), please visit the HSDB record page. Metabolism / Metabolites Extensively metabolized by hepatic cytochrome P450 2D6 isozyme to 9-hydroxyrisperidone (i.e. [paliperidone]), which has approximately the same receptor binding affinity as risperidone. Hydroxylation is dependent on debrisoquine 4-hydroxylase and metabolism is sensitive to genetic polymorphisms in debrisoquine 4-hydroxylase. Risperidone also undergoes N-dealkylation to a lesser extent. Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme, CYP 2D6. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone. CYP 2D6, also called debrisoquin hydroxylase, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP 2D6 is subject to genetic polymorphism (about 6%-8% of Caucasians, and a very low percentage of Asians, have little or no activity and are "poor metabolizers") and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP 2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP 2D6 metabolizers convert it much more slowly. Although extensive metabolizers have lower risperidone and higher 9-hydroxyrisperidone concentrations than poor metabolizers, the pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, are similar in extensive and poor metabolizers. Risperidone has known human metabolites that include 9-Hydroxy-risperidone, Paliperidone, 3-[2-[4-(6-fluoro-2-hydroxy-1,2-benzoxazol-2-ium-3-yl)piperidin-1-yl]ethyl]-2,9-dimethyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one, 3-ethyl-2,9-dimethyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one, and 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole. Extensively metabolized by hepatic cytochrome P450 2D6 isozyme to 9-hydroxyrisperidone, which has approximately the same receptor binding affinity as risperidone. Hydroxylation is dependent on debrisoquine 4-hydroxylase and metabolism is sensitive to genetic polymorphisms in debrisoquine 4-hydroxylase. Risperidone also undergoes N-dealkylation to a lesser extent. Route of Elimination: Risperidone is extensively metabolized in the liver.In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives were prolonged compared to young healthy subjects. Half Life: 20-24 hours Biological Half-Life 3 hours in extensive metabolizers Up to 20 hours in poor metabolizers The apparent half-life of risperidone plus 9-hydroxyrisperidone following Risperdal Consta administration is 3 to 6 days, and is associated with a monoexponential decline in plasma concentrations. This half-life of 3-6 days is related to the erosion of the microspheres and subsequent absorption of risperidone. The apparent half-life of risperidone was 3 hours (CV=30%) in extensive metabolizers and 20 hours (CV=40%) in poor metabolizers. The apparent half-life of 9-hydroxyrisperidone was about 21 hours (CV=20%) in extensive metabolizers and 30 hours (CV=25%) in poor metabolizers. The pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, were similar in extensive and poor metabolizers, with an overall mean elimination half-life of about 20 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Blockade of dopaminergic D2 receptors in the limbic system alleviates positive symptoms of schizophrenia such as hallucinations, delusions, and erratic behavior and speech. Blockade of serotonergic 5-HT2 receptors in the mesocortical tract, causes an excess of dopamine and an increase in dopamine transmission, resulting in an increase in dopamine transmission and an elimination of core negative symptoms. Dopamine receptors in the nigrostriatal pathway are not affected by risperidone and extrapyramidal effects are avoided. Like other 5-HT2 antagonists, risperidone also binds at alpha(1)-adrenergic receptors and, to a lesser extent, at histamine H1 and alpha(2)-adrenergic receptors. Toxicity Data LD50=82.1mg/kg (orally in mice). Interactions Given the primary CNS effects of risperidone, caution should be used when Risperdal is taken in combination with other centrally-acting drugs and alcohol. Risperdal may antagonize the effects of levodopa and dopamine agonists. When Risperdal is co-administered with enzyme inducers (e.g., carbamazepine), the dose of Risperdal should be increased up to double the patient's usual dose. It may be necessary to decrease the Risperdal dose when enzyme inducers such as carbamazepine are discontinued [see Drug Interactions (7.1)]. Similar effect may be expected with co-administration of Risperdal with other enzyme inducers (e.g., phenytoin, rifampin, and phenobarbital). Chronic administration of clozapine with Risperdal may decrease the clearance of risperidone. For more Interactions (Complete) data for RISPERIDONE (10 total), please visit the HSDB record page. Hepatotoxicity Liver test abnormalities may occur in up to 30% of patients on long term therapy with risperidone, usually arising within the first 8 weeks of treatment. The ALT elevations are usually mild, transient and may resolve even with continuation of medication. Instances of more marked ALT and alkaline phosphatase elevations, with or without symptoms and with or without jaundice, have also been reported. The onset of injury typically occurs within a few days of starting risperidone and resolves rapidly with stopping. Instances of acute liver injury with jaundice arising several months and even years after starting risperidone have also been reported. The pattern of serum enzyme elevations is typically cholestatic, but cases with hepatocellular and mixed patterns have also been described. Immunoallergic manifestations (rash, fever, eosinophilia) are rare; a case of autoimmune hepatitis apparently triggered by risperidone therapy has been published, but most cases do not have autoimmune features. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal risperidone doses of up to 6 mg daily produce low levels in milk. Sedation, failure to thrive, jitteriness, tremors, abnormal muscle movements and respiratory depression have been reported in infants exposed to risperidone in milk. Because there is little published experience with risperidone during breastfeeding and little long-term follow-up data, other agents may be preferred, especially while nursing a newborn or preterm infant. Systematic reviews of second-generation antipsychotics concluded that risperidone seemed to be a second-line agent during breastfeeding because of the limited data available and higher excretion into milk relative to other agents. A safety scoring system finds risperidone to be possible to use cautiously during breastfeeding. Monitor the infant for drowsiness, weight gain, tremors, respiratory rate, abnormal muscle movements, and developmental milestones, especially if other antipsychotics are used concurrently. ◉ Effects in Breastfed Infants One woman took risperidone 4 mg daily during breastfeeding. Her infant showed no developmental abnormalities on examinations up to 9 months of age. Another mother took risperidone 6 mg daily during breastfeeding. Her infant showed no developmental abnormalities on examinations up to 12 months of age. Two women taking risperidone 4 mg and 1.5 mg daily breastfed their infants of 3.3 months and 6 weeks of age, respectively, were achieving normal developmental milestones and had no adverse effects reported. A 1 week postpartum woman was started on risperidone 2 mg daily and increased after 10 days to a dosage of 3 mg daily. She breastfed her infant 6 times daily. The infant was observed for 5 weeks of inpatient therapy and judged normal by a pediatric neurologist. No sedation or other adverse effects were observed in the infant. After 3 months of treatment with risperidone, the mother and infant were judged to be well. An infant had been exclusively breastfed for 3 months during maternal therapy with risperidone 1 mg daily. A pediatric examination found the infant to have no neurological or physical abnormalities, and appeared to interact appropriately. In a telephone follow-up study, 124 mothers who took a benzodiazepine while nursing reported whether their infants had any signs of sedation. One mother who was taking 0.75 mg of risperidone daily, flurazepam 15 mg daily, clonazepam 0.25 mg twice daily, and 1 mg of bupropion daily reported sedation in her breastfed infant. A woman diagnosed with schizophrenia was taking risperidone 1.5 mg daily during late pregnancy and postpartum while nursing (extent not stated) her full-term infant. At 2 weeks postpartum, haloperidol 0.8 mg daily was added because of a recurrence of symptoms. At these dosages, no adverse effects were seen in the infant. However, because of recurring symptoms, the dosage of haloperidol was increased to 1.5 mg daily. Three days later, the infant had excessive sedation, poor feeding, and slowing in motor movements. Pediatric assessment found no medical reason for these effects. Breastfeeding was discontinued and the infant's symptoms resolved completely in 5 days. The infant's symptoms were probably caused by the drug combination. A prospective cohort study of infants breastfed by mothers in an inpatient mother-baby psychiatric unit in India followed 7 infants who were exposed to risperidone in breastmilk; most received partial supplementation. One infant whose mother was taking risperidone 4 mg and lorazepam 2 mg developed sedation that resolved when lorazepam was discontinued. One infant whose mother received risperidone 4 mg daily, trihexyphenidyl 2 mg daily, and electroconvulsive therapy developed constipation. Infants were followed for 1 to 3 months after discharge. One infant had delayed weight development, one infant had delay in height, one infant mental delay, and a fourth infant had motor and mental delay. A woman with bipolar disorder was maintained on oral risperidone 2 mg at bedtime, long-acting injectable risperidone 50 mg intramuscular every 2 weeks, oral citalopram 20 mg daily, and oral benztropine 0.5 mg daily. She became pregnant and maintained the same regimen. Her infant was born at 35 weeks gestational age and was breastfed (extent and duration not stated). At 16 months of age, the infant was doing well and met his developmental milestones. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who were not treated with a second-generation antipsychotic (n = 818). Of the patients who were taking a second-generation antipsychotic drug, 60.4% were on more than one psychotropic. A review of the pediatric medical records, no adverse effects were noted among infants exposed or not exposed to second-generation antipsychotic monotherapy or to polytherapy. The number of women taking risperidone was not reported. A preterm infant weighing 2.75 kg was born at 35 weeks gestation. The infant received bag and mask ventilation for 2 min and was kept on oxygen for the first 18 hours of life due to respiratory distress. The baby began breastfeeding on day 2 of life. On day 12, the mother was started on risperidone 1 mg daily for psychotic episodes. On day 13, the infant developed a respiratory rate of 16/min and no retractions and was placed on CPAP for 12 hours, with gradual weaning thereafter and was placed on formula. On day 15, the mother began breastfeeding again and the respiratory depression recurred. Feeding was changed to breastmilk expressed prior to the daily dose of risperidone and formula for 6 hours after each dose followed by direct breastfeeding. Over the next 2 days no further episodes of respiratory depression occurred. The baby was discharged on day 24, with advice to continue the same feeding pattern. Respiratory depression was probably caused by risperidone in milk. A woman diagnosed with undifferentiated schizophrenia took risperidone 4 to 5 mg and trihexyphenidyl 2 mg daily throughout 5 pregnancies. She breastfed each infant for 20 to 24 months. No adverse developmental consequences were noted in any of the children. At the time of publication, the oldest three children, aged 26, 23 and 22 years, had completed their education and were employed, while the youngest two were 15 and 19 years old and were doing well academically in their education. ◉ Effects on Lactation and Breastmilk Risperidone has caused elevated prolactin serum levels, gynecomastia, and galactorrhea in patients taking the drug. In one case, euprolactinemic gynecomastia and galactorrhea occurred in a 19-year-old man who was also taking fluvoxamine. A meta-analysis of 3 studies found that the risk of gynecomastia with risperidone is 4.3 times greater than that of quetiapine. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Patients enlisted in the National Pregnancy Registry for Atypical Antipsychotics who were taking a second-generation antipsychotic drug while breastfeeding (n = 576) were compared to control breastfeeding patients who primarily had diagnoses of major depressive disorder and anxiety disorders, most often treated with SSRI or SNRI antidepressants, but not with a second-generation antipsychotic (n = 818). Among women on a second-generation antipsychotic, 60.4% were on more than one psychotropic compared with 24.4% among women in the control group. Of the women on a second-generation antipsychotic, 59.3% reported “ever breastfeeding” compared to 88.2% of women in the control group. At 3 months postpartum, 23% of women on a second-generation antipsychotic were exclusively breastfeeding compared to 47% of women in the control group. The number of women taking risperidone was not reported. ◈ What is risperidone? Risperidone is a medication that has been used to treat mental health conditions such as schizophrenia, bipolar disorder, and depression. It can be taken by mouth or given as an injection. Risperidone belongs to a group of medications called atypical or second-generation antipsychotics. Brand names for risperidone include Risperdal®, Risperdal Consta®, and Perseris®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take risperidone. Can it make it harder for me to get pregnant? In some people, risperidone may raise the levels of a hormone called prolactin. High levels of prolactin can stop ovulation (part of the menstrual cycle when an ovary releases an egg). This would make it harder to get pregnant. Your healthcare provider can test your levels of prolactin if there is concern. ◈ Does taking risperidone increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Based on the studies reviewed, risperidone is not expected to increase the chance of miscarriage. ◈ Does taking risperidone increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Based on the studies reviewed, risperidone is not expected to increase the chance of birth defects above the background risk. ◈ Does taking risperidone in pregnancy increase the chance of other pregnancy-related problems? Based on the studies reviewed, risperidone may cause low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth).Risperidone may cause weight gain and problems with blood sugar in a person who is pregnant. This may increase the chance of developing gestational diabetes. More information about gestational diabetes can be found in our fact sheet https://mothertobaby.org/fact-sheets/diabetes-pregnancy/. ◈ I need to take risperidone throughout my entire pregnancy. Will it cause withdrawal symptoms in my baby after birth? The use of some medications during pregnancy may cause temporary symptoms in newborns soon after birth. These symptoms are sometimes referred to as withdrawal. It is unknown if taking risperidone alone could increase the chance of withdrawal symptoms in a newborn. Similar medications have been associated with a chance for withdrawal, so babies exposed to risperidone near the time of delivery should be watched for stiff or floppy muscles, drowsiness, agitation, tremors, trouble breathing, and problems with feeding. In most cases, symptoms would be expected to go away in a few days without any long-term health effects. It is important that your healthcare providers know you are taking risperidone so that if symptoms occur your baby can get the care that is best for them. ◈ Does taking risperidone in pregnancy affect future behavior or learning for the child? Studies have not been done to see if risperidone can cause behavior or learning issues for the child. ◈ Breastfeeding while taking risperidone: Information on the use of risperidone during breastfeeding is limited. When taken in doses of up to 6 mg a day risperidone was found in breastmilk in small amounts. Side effects were not reported in a small number of breastfed infants who were exposed to risperidone only (in doses of up to 6 mg a day). If you take risperidone and other medications, there may be a higher chance for side effects in the baby. If you suspect the baby has any symptoms (sleepiness, poor feeding, crankiness, or unusual movements) contact the child’s healthcare provider.The product label for risperidone recommends that people who are breastfeeding not use this medication. But the benefit of using risperidone may outweigh the possible risks. Your healthcare providers can talk with you about using risperidone and what treatment is best for you. Be sure to talk to your healthcare provider about all of your breastfeeding questions. ◈ If a male takes risperidone, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? Using risperidone may raise a person’s levels of the hormone prolactin, which may affect fertility. Studies have not been done to see if risperidone could increase the chance of birth defects above the background risks. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antipsychotic Agents; Dopamine Antagonists; Serotonin Antagonists Risperdal (risperidone) is indicated for the treatment of schizophrenia. Efficacy was established in 4 short-term trials in adults, 2 short-term trials in adolescents (ages 13 to 17 years), and one long-term maintenance trial in adults /Included in US product label/ Risperdal adjunctive therapy with lithium or valproate is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in one short-term trial in adults. /Included in US product label/ Risperdal is indicated for the treatment of irritability associated with autistic disorder, including symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods. Efficacy was established in 3 short-term trials in children and adolescents (ages 5 to 17 years). /Included in US product label/ Risperdal is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in 2 short-term trials in adults and one short-term trial in children and adolescents (ages 10 to 17 years). /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Risperdal (risperidone) is not approved for the treatment of patients with dementia-related psychosis. Like other antipsychotic agents (e.g., phenothiazines), risperidone has been associated with tardive dyskinesias. Although it has been suggested that atypical antipsychotics appear to have a lower risk of tardive dyskinesia, whether antipsychotic drugs differ in their potential to cause tardive dyskinesia is as yet unknown. In one open-label study, an annual incidence of tardive dyskinesia of 0.3% was reported in patients with schizophrenia who received approximately 8-9 mg of oral risperidone daily for at least 1 year. The prevalence of this syndrome appears to be highest among geriatric patients (particularly females). The risk of developing tardive dyskinesia and the likelihood that it will become irreversible also appear to increase with the duration of therapy and cumulative dose of antipsychotic agents administered; however, the syndrome may occur, although much less frequently, after relatively short periods of treatment with low dosages. Neuroleptic malignant syndrome (NMS), a potentially fatal symptom complex, has been reported in patients receiving antipsychotic agents. NMS requires immediate discontinuance of the drug and intensive symptomatic and supportive care. Dose-related somnolence was a commonly reported adverse effect associated with risperidone treatment. Approximately 8% of adult patients with schizophrenia receiving 16 mg of oral risperidone daily and 1% of patients receiving placebo reported somnolence in studies utilizing direct questioning or a checklist to detect adverse events, respectively. For more Drug Warnings (Complete) data for RISPERIDONE (41 total), please visit the HSDB record page. Pharmacodynamics The primary action of risperidone is to decrease dopaminergic and serotonergic pathway activity in the brain, therefore decreasing symptoms of schizophrenia and mood disorders. Risperidone has a high binding affinity for serotonergic 5-HT2A receptors when compared to dopaminergic D2 receptors in the brain. Risperidone binds to D2 receptors with a lower affinity than first-generation antipsychotic drugs, which bind with very high affinity. A reduction in extrapyramidal symptoms with risperidone, when compared to its predecessors, is likely a result of its moderate affinity for dopaminergic D2 receptors. |
| 分子式 |
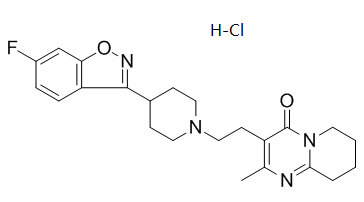
C₂₃H₂₈CLFN₄O₂
|
|---|---|
| 分子量 |
446.95
|
| 精确质量 |
446.188
|
| CAS号 |
666179-74-4
|
| 相关CAS号 |
Risperidone; 106266-06-2; Risperidone mesylate; 666179-96-0
|
| PubChem CID |
9889802
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
61.9
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
731
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl[H].FC1C([H])=C([H])C2=C(C=1[H])ON=C2C1([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C2=C(C([H])([H])[H])N=C3C([H])([H])C([H])([H])C([H])([H])C([H])([H])N3C2=O)C([H])([H])C1([H])[H]
|
| InChi Key |
OCBZQKQWVUTYDN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H27FN4O2.ClH/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22;/h5-6,14,16H,2-4,7-13H2,1H3;1H
|
| 化学名 |
3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one;hydrochloride
|
| 别名 |
R 64 766 hydrochloride; Risperidone hydrochloride; 666179-74-4; Risperidone (hydrochloride); R 64 766 hydrochloride; SCHEMBL5774262; OCBZQKQWVUTYDN-UHFFFAOYSA-N; R-64 766; R64766
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2374 mL | 11.1869 mL | 22.3739 mL | |
| 5 mM | 0.4475 mL | 2.2374 mL | 4.4748 mL | |
| 10 mM | 0.2237 mL | 1.1187 mL | 2.2374 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04940039 | Active Recruiting |
Drug: Risperidone 3 mg Drug: Paliperidone Palmitate 50 mg eq. |
Schizophrenia | Janssen-Cilag International NV | July 22, 2021 | Phase 4 |
| NCT03522168 | Active Recruiting |
Drug: Risperidone Drug: Aripiprazole |
Weight, Body | Duke University | January 10, 2019 | N/A |
| NCT05480046 | Recruiting | Drug: Risperidone ISM | Schizophrenia | Rovi Pharmaceuticals Laboratories | October 18, 2022 | N/A |
| NCT05779241 | Recruiting | Drug: LYN-005 Drug: Risperidone |
Schizophrenia Schizoaffective Disorder |
Lyndra Inc. | April 2023 | Phase 3 |
| NCT05890768 | Recruiting | Drug: Lumateperone Drug: Risperidone |
Psychosis | University of New Mexico | May 11, 2023 | Phase 4 |
|
|
|