| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
PDE4 (IC50 = 0.2~0.9 nM)
|
|---|---|
| 体外研究 (In Vitro) |
罗氟司特/Roflumilast是大多数测试的 PDE4 剪接变体的亚纳摩尔抑制剂,并且对 PDE4 以外的 PDE 酶没有影响。除了 PDE4C(4C1,IC50=3 nM;4C2,IC50= 4.3 nM)外,其抑制效力稍差,它没有表现出 PDE4 同工型选择性 [2]。罗氟司特是一种强效且特异性的 PDE4 抑制剂。在浓度高达 10,000 倍时,罗氟司特不会影响其他 PDE 同工酶,例如 PDE1、PDE2、PDE3 或 PDE5。这使其成为 PDE4 的单选择性抑制剂。罗氟司特抑制人中性粒细胞的活性。罗氟司特可阻止单核细胞衍生的树突状细胞合成 TNFα。 rolfumilast 抑制细胞因子合成和 CD4+ T 细胞增殖。 rolumilast 在 7 nM 的效力 (IC30) 下可抑制高达 60% 的增殖 [3]。
1993年,在一项综合筛选规划中,从一系列苯酰胺中鉴定出罗氟司特。Roflumilast/罗氟司特的高效和选择性竞争性抑制PDE4,而不影响PDE1、2、3或5同工酶,来自各种细胞和组织,此前已有报道。这些早期研究已经扩展到一系列人类重组PDE酶对PDE1-11的测试(表1)。结果证实罗氟司特不影响任何其他PDE酶,并且是大多数PDE4剪接变体的亚纳摩尔抑制剂。罗氟司特对PDE4亚型没有选择性,但对PDE4C有抑制作用,效价略低。PDE4亚型抑制的一半最大抑制浓度(IC50)值(表1)在其他人报道的截断PDE4A-D版本的抑制范围内[1]。 从一系列苯酰胺衍生物中,Roflumilast(3-环丙基甲氧基-4-二氟甲氧基- n -[3,5-二氯吡啶-4-基]-苯酰胺)被鉴定为一种有效的选择性PDE4抑制剂。它抑制人中性粒细胞PDE4的活性,IC(50)为0.8 nM,即使在10,000倍的浓度下也不影响PDE1(牛脑),PDE2(大鼠心脏)和PDE3和PDE5(人血小板)。罗氟米司特在体内形成的主要代谢物(罗氟米司特n -氧化物)和piclamilast (RP 73401)的效力几乎相同,但比罗利普兰和Ariflo (cilomilast;某人207499)。利用细胞特异性反应研究了罗氟司特及其参比化合物在各种人类白细胞中的抗炎和免疫调节潜能:中性粒细胞[n-甲酰基-甲基-leucyl-苯丙氨酸(fMLP)诱导的LTB(4)和活性氧(ROS)的形成],嗜酸性粒细胞(fMLP-和c5a诱导的ROS的形成),单核细胞,单核细胞来源的巨噬细胞,树突状细胞(脂多糖诱导的肿瘤坏死因子α合成),和CD4+ T细胞(抗cd3 /抗cd28单克隆抗体刺激的增殖,IL-2, IL-4, IL-5和干扰素γ释放)。与细胞类型和所研究的反应无关,罗氟司特相应的IC值(半最大抑制)在一个狭窄的范围内(2-21 nM),与罗氟司特n -氧化物(3-40 nM)和piclamilast (2-13 nM)非常相似。相比之下,西洛司特(40-3000 nM)和罗利普兰(10-600 nM)对中性粒细胞的效价最高,差异更大。与代表终末炎症效应细胞的中性粒细胞和嗜酸性粒细胞相比,罗氟司特及其n -氧化物对单核细胞、CD4+ T细胞和树突状细胞的相对效力明显高于西罗司特和罗利普兰,这可能反映了免疫调节潜力的改善。罗氟米司特在体外和体内的疗效(见本期随附文章)表明,罗氟米司特将有助于治疗慢性炎症性疾病,如哮喘和慢性阻塞性肺病。[3] |
| 体内研究 (In Vivo) |
对动物的研究表明,使用罗氟司特可减少小鼠、大鼠或豚鼠短期接触烟草烟雾后支气管肺泡灌洗液中中性粒细胞的积聚;此外,它还能消除暴露于烟草烟雾七个月的大鼠肺实质中炎症细胞的浸润[2]。在 pIgR 中,rolumilast 可以预防 COPD 的进展?*?老鼠。 9 个月大的 WT 或 pIgR 用于这些研究?*?三个月内,小鼠接受 100 μg 罗氟司特 (5 μg/g) 或载体(4% 甲基纤维素,1.3% PEG400)口服强饲治疗。大约12个月大时,肺部被摘除。当罗氟司特给予小鼠时,轻微气道壁重塑并未像媒介物处理的 pIgR-/- 动物那样进展。令人惊讶的是,12 个月大的 pIgR 接受了罗鲁司特?*?与9个月大的pIgR相比,小鼠的肺气肿指数较低。*?正如小鼠所证明的那样,在这种情况下,罗氟司特不仅可以阻止肺气肿的发展。似乎有助于解决整个肺气肿过程中肺实质的肺气肿损失[4]。
在体内实验中,罗氟司特能显著改善COPD关键病理机制,包括烟草烟雾诱导的肺部炎症、黏液纤毛功能障碍、肺纤维化与肺气肿样重塑、氧化应激、肺血管重塑及肺动脉高压。体外研究显示,罗氟司特N-氧化物可影响多种细胞功能,包括中性粒细胞、单核/巨噬细胞、CD4+与CD8+ T细胞、内皮细胞、上皮细胞、平滑肌细胞及成纤维细胞。这些细胞效应被认为是罗氟司特改善COPD病理机制的基础,最终体现为急性加重减少和肺功能改善。作为一种多组分疾病,COPD需要PDE4抑制实现的广谱治疗策略。但需注意,作为PDE4抑制剂,罗氟司特并非直接支气管扩张剂。[1] 罗氟司特阻断pIgR−/−小鼠的COPD进展[4] 为探究pIgR−/−小鼠进行性小气道重塑和肺气肿是否由细菌诱导的炎症驱动,我们使用磷酸二酯酶-4抑制剂罗氟司特进行抗炎干预。该药已获FDA批准用于COPD患者,并在小鼠COPD模型中证实具有抗炎作用42,43,44,45。本研究对9月龄WT或pIgR−/−小鼠每日灌胃给予100μg罗氟司特(5μg·g−1)或溶媒(4%甲基纤维素,1.3% PEG400)持续3个月,于12月龄取肺组织。与溶媒组不同,罗氟司特治疗完全阻止了小气道壁重塑的进展(图6a)。值得注意的是,治疗组12月龄pIgR−/−小鼠较9月龄小鼠肺气肿指标反而降低,表明该药不仅阻断疾病进展,还可能促进肺实质气肿样破坏的修复(图6b,c)。与无菌环境饲养小鼠类似,罗氟司特处理的WT和pIgR−/−小鼠肺实质中性粒细胞极少(图6d),巨噬细胞数量与溶媒处理的WT小鼠相当(图6e)。伴随炎症减轻,pIgR−/−小鼠肺组织MMP-12和NE表达下降(图6f和附图6)。此外,与溶媒组相比,治疗组肺组织NF-κB活化和KC表达降低(图6g,h和附图7)。这些数据共同表明,持续细菌性炎症驱动了pIgR−/−小鼠的COPD样重塑。 高脂饮食(HFD)大鼠体重显著增加且不受罗氟司特治疗影响。尿动力学检测显示肥胖大鼠排尿频率和非排尿收缩增加,而罗氟司特可逆转这些异常。这些改变伴随逼尿肌中TNF-α、IL-6、IL-1β和NF-κB表达显著升高。罗氟司特能降低逼尿肌炎症因子表达。 结论:罗氟司特口服治疗可恢复HFD喂养大鼠的正常膀胱功能,并下调膀胱炎症因子表达。[5] 罗氟司特/Roflumilast对肥胖大鼠膀胱功能障碍的改善作用[5] 为期4周的口服罗氟司特治疗显著改善肥胖大鼠膀胱功能参数:膀胱容量(0.54±0.08ml;N=10)、排尿量(0.52±0.08ml;N=10)、排尿间隔(2.8±0.4min;N=10)及非排尿收缩频率(0.7±0.4;N=10),均优于HFD+溶媒组(N=10;P<0.05;图1)。但最大排尿压力在治疗组(41.5±6.8cm H2O,N=10;P>0.05)无显著变化(图1a,d)。治疗后尿动力学参数与正常饮食+溶媒组相当(N=10;P>0.05;图1)。结果表明罗氟司特可有效改善肥胖大鼠膀胱功能及排尿效率。 罗氟司特抑制肥胖大鼠炎症反应[5] 为验证该PDE4抑制剂是否通过抑制炎症反应改善肥胖大鼠逼尿肌过度活动(DO),我们对实验动物进行口服罗氟司特干预。免疫组化显示,与溶媒处理的肥胖大鼠相比,治疗组膀胱平滑肌炎症细胞因子表达显著降低(NF-κB 0.68±0.06,TNF-α 0.41±0.06,IL-6 0.39±0.09,IL-1β 0.36±0.09;灰度值;n=16;P<0.05;图2)。qRT-PCR进一步证实治疗组炎症因子基因表达下调(NF-κB 0.64±0.08,TNF-α 0.39±0.08,IL-6 0.37±0.09,IL-1β 0.41±0.09;n=16;P<0.05;图3),且表达水平与正常饮食+溶媒组无差异(n=16;P>0.05;图2,3)。因此,PDE4抑制剂可能通过抑制炎症介质释放和免疫细胞活化发挥治疗作用。 先将Roflumilast/罗氟司特溶于碱性溶液(0.1 N NaOH)中,用0.1 N HCl滴定至pH 7.4,再用生理盐水稀释 动物在实验前一周在实验室适应,然后随机分为五组(每组10只大鼠),饲养在单独的聚碳酸酯笼中。组(1)仅接受Vehicle(PBS和0.8%甲基纤维素)。组(2)给予Roflumilast/罗氟司特(1 mg/kg, P.O.),每日1次,连用7天,加PBS溶液0.5 ml (i.p)。组(3)单次注射CIS,剂量为7 mg/kg, i.p (Rezvanfar et al., 2013)。加上0.8%的甲基纤维素(P.O)。4组给予 0.3 mg/kg剂量的Roflumilast/罗氟司特,在给药前30 min口服灌胃,连续7天。组(5)给予Roflumilast,剂量为1 mg/kg,在CIS给药前30 min口服灌胃,连续7天。/罗氟司特给药的剂量和途径是根据先前报道的研究选择的[6]。 |
| 酶活实验 |
生化测定[6]
氧化应激参数测定[6] 睾丸匀浆中丙二醛(MDA)(脂质过氧化的标志物)、一氧化氮和谷胱甘肽的含量分别根据Preuss等人(1998)、Grisham等人(1996)和Griffith(1980)描述的方法测定。睾丸CAT活性根据制造商的说明使用商业检测试剂盒进行测定。 细胞内cAMP测定[6] 使用cAMP酶免疫测定(ELISA)试剂盒,按照制造商的说明,测量睾丸匀浆中细胞内cAMP水平。 camp依赖性蛋白激酶(PKA)和HO-1活性测定[6] 睾丸组织PKA活性测定采用Abcam匀浆PKA激酶活性测定试剂盒。该试剂盒是一种灵敏、安全、无放射性的酶联免疫吸附试验,提供了一种快速可靠的方法来定量PKA的活性,该方法利用一种特定的合成肽作为PKA的底物,并使用一种多克隆抗体来识别底物的磷酸化形式。所有的程序都是按照制造商的说明完成的。 为了测定HO-1的活性,睾丸匀浆样品在血红素(50 mmol/L)、大鼠肝细胞质(5 mg/mL)、MgCl2 (2 mmol/L)、葡萄糖-6-磷酸(2 mmol/L)、葡萄糖-6-磷酸脱氢酶(1单位)和烟酰胺腺嘌呤二核苷酸磷酸(NADPH) (0.8 mmol/L)的混合物中,在0.5 mL PBS (pH 7.4)中,37℃孵育60 min。将试管浸入冰中冷却,停止反应。提取胆红素产物,在520 nm处分光光度法测定其浓度,利用消光系数法计算其浓度(Abraham et al., 1988)。 促炎细胞因子及凋亡标志物测定[6] 睾丸组织匀浆中白细胞介素-1β (IL-1β)、肿瘤坏死因子α (TNF-α)和凋亡标志物Bax和Bcl-2的水平,采用R&D Systems购买的特异性大鼠ELISA试剂盒进行检测。此外,按照标准制造商的说明,用比色试剂盒测量Caspase-3的活性。 总蛋白测定[6] 采用Lowry的方法(Waterborg, 2009)测定睾丸组织匀浆内总蛋白浓度,以牛血浆白蛋白为标准品。 |
| 细胞实验 |
细胞毒性研究[6]
采用磺酰罗丹明B(SRB)法(Skehan等,1990)检测细胞毒性。将癌细胞接种于96孔平底培养板中培养24小时,随后更换为含不同浓度药物的新鲜培养基。分别加入梯度浓度(0、1、5、10、25和50 mg/mL)的待测药物顺铂(CIS)和罗氟司特,于37℃作用48小时。每个剂量设三个复孔,用于计算各药物的半数抑制浓度(IC50,即抑制50%细胞生长所需的药物浓度)。 在另一实验中,将顺铂IC50(3.9 mg/mL)与罗氟司特IC50(2.3 mg/mL)联合作用于细胞,测定细胞存活率%与抑制率%。罗氟司特及其他化合物先用二甲基亚砜(DMSO)溶解,再用培养基稀释至工作浓度。所有处理组中DMSO终浓度不超过0.1%(v/v),该浓度对细胞活性无显著影响。药物处理后,细胞经4℃预冷的10%三氯乙酸固定1小时,蒸馏水洗涤后,用0.4% SRB(溶于1%乙酸)于25℃染色30分钟。最后用10 mM三羟甲基氨基甲烷缓冲液(pH 10.5)溶解染料,于564 nm波长处测定吸光度。根据细胞存活分数与药物浓度的关系曲线计算IC50值。 实时定量PCR[6] 采用β-肌动蛋白作为内参基因,通过实时定量PCR检测罗氟司特对顺铂诱导的大鼠睾丸组织及PC3癌细胞系中信号转录因子和凋亡标志物基因表达的影响。 体内研究部分:使用TRIzol试剂盒提取睾丸组织总RNA,经RNeasy纯化试剂盒纯化后,于260 nm测定浓度。体外细胞毒性研究部分:按前述方法培养PC3前列腺癌细胞系,24小时后更换为含下列处理的新鲜培养基:顺铂IC50(3.9 mg/mL)、罗氟司特IC50(4.7 mg/mL)或两药联用,37℃处理48小时。处理后收集细胞,用冰PBS洗涤后进行裂解。 |
| 动物实验 |
Roflumilast administration [4]
For studies using roflumilast, 200 μl of 0.5 mg ml−1 suspension of Roflumilast or vehicle (4% methylcellulose, 1.3% PEG400 and ∼5 μg drug per mg animal weight) was administered by oral gavage once daily, 5 days a week for the duration of treatment. The roflumilast suspension was freshly prepared each week and stored at 4 °C. Diet-induced obesity and study treatment [5] For 12 weeks, study animals were housed three per cage on a 12-h light–dark cycle, and either normal diet (ND) (fat: 5%; protein: 20%; carbohydrate: 75%) or HFD (fat: 30%; protein: 14%; carbohydrate: 56%) that induces obesity as previously described [17, 18]. Study animals were divided into three groups (N = 30 in each group): (1) vehicle-treated ND-fed (ND + vehicle) rats (normal diet for 8 weeks before receiving the vehicle); (2) vehicle-treated HFD-fed (HFD + vehicle) rats (HFD for 8 weeks before receiving the vehicle); and (3) Roflumilast-treated HFD-fed (HFD + roflumilast) rats (HFD for 8 weeks before receiving roflumilast). Roflumilast (5 mg/kg/day) or vehicle (sterile water used as solvent for roflumilast) was administered orally by gavage during the last 4 weeks of HFD or ND feeding. All rats were weighed at 12 weeks, and urodynamic studies were conducted in ten rats of each group. Study animals were then killed in a carbon dioxide tank prior to collection of bladder specimens; the bladder mucosa was separated under microscopy, and the DSM tissue was preserved in liquid nitrogen. Methods: In this 12-week study, 90 female Sprague-Dawley rats were divided into three groups: (1) vehicle-treated normal diet (ND)-fed rats; (2) vehicle-treated high-fat diet (HFD)-fed rats; and (3) Roflumilast-treated HFD-fed rats. Oral roflumilast (5 mg/kg/day) was administered during the last 4 weeks of HFD feeding in the test group. At 12 weeks, a urodynamic study was performed in ten rats of each group. Bladder tissue was extracted, the bladder mucosa was separated under microscopy, and bladder detrusor smooth muscle (DSM) expression of TNF-α, interleukin (IL)-6, IL-1β, and nuclear factor kappa B (NF-κB) were analyzed using Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) [5]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After a 500mcg dose, the bioavailability of Roflumilast is about 80%. In the fasted state, maximum plasma concentrations are reached in 0.5 to 2 hours, while in the fed state, Cmax is reduced by 40%, Tmax is increased by one hour, and total absorption is unchanged. Applied topically, the mean systemic exposure for roflumilast and its N-oxide metabolite in adults was 72.7 ± 53.1 and 628 ± 648 h∙ng/mL, respectively. The mean systemic exposure for roflumilast and its N-oxide metabolite in adolescents was 25.1 ± 24.0 and 140 ± 179 h∙ng/mL, respectively. Roflumilast is excreted 70% in the urine as roflumilast N-oxide. Following a single oral dose of 500 mcg, the volume of distribution of roflumilast is approximately 2.9 L/kg. Plasma clearance of roflumilast following short-term intravenous infusion is approximately 9.6 L/h. Metabolism / Metabolites Roflumilast is metabolized to roflumilast N-oxide, the active metabolite of roflumilast in humans, by CYP3A4 and CYP1A2. The N-oxide metabolite is less potent than its parent drug in regards to PDE4 inhibition, but its plasma AUC is approximately 10-fold greater. Biological Half-Life Following oral administration, the plasma half-lives of roflumilast and roflumilast N-oxide are 17 hours and 30 hours, respectively. Roflumilast is rapidly metabolised to its N-oxide at the dichloropyridyl moiety by cytochrome P450 (CYP) 3A4 and CYP1A2 enzymes (Fig. 1A). As indicated in Table 1, roflumilast N-oxide is only 2–3-fold less potent than the parent compound with respect to PDE4 inhibition, maintains high selectivity to other PDE isoenzymes and shows no selectivity for PDE4 subtypes. In man, this metabolite is estimated to account for about 90% of the overall PDE4 inhibition and 10% is attributed to the roflumilast parent. On repeated oral dosing with roflumilast 500 μg once daily in healthy subjects, the free drug concentration of roflumilast N-oxide in plasma over 24 h was estimated to be about 1–2 nM by considering the plasma protein binding of roflumilast N-oxide of approximately 97% (Fig. 1B). Although it is well-known that tobacco smoking increases CYP1A2, it was found that smoking has a minor influence on the pharmacokinetic profile of roflumilast. Before information was available for roflumilast, intriguing clinical results were reported in COPD and asthma for cilomilast, an extensively characterised PDE4 inhibitor. However, no further development of cilomilast has been reported recently. Unlike roflumilast and its N-oxide, cilomilast shows some subtype selectivity for PDE4D (Table 1). This may be a disadvantage in terms of adverse events, as PDE4D may be linked to emesis and/or cardiovascular side-effects in patients at risk of heart failure [1]. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration studies, roflumilast was not associated with serum enzyme elevations or with episodes of clinically apparent liver injury. Since approval of roflumilast, there have been no published reports of hepatotoxicity, and the product label does not mention liver injury as an adverse event. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of roflumilast in nursing mothers. The drug and its metabolite are more than 97% bound to plasma proteins, so amounts in milk are likely to be very low. However, the manufacturer and some experts recommend that the oral drug should not be used by women who are nursing. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding of roflumilast and its N-oxide metabolite is approximately 99% and 97%, respectively. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Roflumilast and its active metabolite, roflumilast N-oxide, increase cyclic adenosine-3′, 5′-monophosphate (cAMP) in affected cells by inhibiting PDE4. They are highly selective for PDE4 and are effectively inactive against PDEs 1, 2, 3, 5, and 7. Roflumilast is a benzamide obtained by formal condensation of the carboxy group of 3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid with the amino group of 3,5-dichloropyridin-4-amine. Used for treatment of bronchial asthma and chronic obstructive pulmonary disease. It has a role as a phosphodiesterase IV inhibitor and an anti-asthmatic drug. It is a member of benzamides, a chloropyridine, an aromatic ether, an organofluorine compound and a member of cyclopropanes. Roflumilast is a highly selective phosphodiesterase-4 (PDE4) inhibitor. PDE4 is a major cyclic-3',5′-adenosinemonophosphate (cyclic AMP, cAMP)-metabolizing enzyme expressed on nearly all immune and pro-inflammatory cells, in addition to structural cells like those of the smooth muscle or epithelium. The resultant increase in intracellular cAMP induced by roflumilast's inhibition of PDE4 is thought to mediate its disease-modifying effects, although its precise mechanism of action has yet to be elucidated. The oral formulation of roflumilast is indicated to manage chronic obstructive pulmonary disease. It was first approved by the EMA in July 2010, and by the FDA in January 2018. Roflumilast topical cream is indicated to treat plaque psoriasis. The cream formulation was first approved by the FDA in July 2022 and by Health Canada in April 2023. On December 15, 2023, the FDA approved a new topical foam formulation of roflumilast for the treatment of seborrheic dermatitis in patients aged 9 years and older. Roflumilast is a Phosphodiesterase 4 Inhibitor. The mechanism of action of roflumilast is as a Phosphodiesterase 4 Inhibitor. Roflumilast is a selective inhibitor of phosphodiesterase-4 (PDE-4) that has unique antiinflammatory activity and is used to treat and prevent exacerbations of chronic obstructive pulmonary disease (COPD). Roflumilast has not been linked to significant serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury. Roflumilast is an orally available, long-acting inhibitor of phosphodiesterase (PDE) type 4 (PDE4), with anti-inflammatory and potential antineoplastic activities. Upon administration, roflumilast and its active metabolite roflumilast N-oxide selectively and competitively bind to and inhibit PDE4, which leads to an increase of both intracellular levels of cyclic-3',5'-adenosine monophosphate (cAMP) and cAMP-mediated signaling. cAMP prevents phosphorylation of spleen tyrosine kinase (SYK) and abrogates activation of the PI3K/AKT/mTOR signaling pathway, which may result in the induction of apoptosis. PDE4, a member of the PDE superfamily that hydrolyses cAMP and 3',5'-cyclic guanosine monophosphate (cGMP) to their inactive 5' monophosphates, is upregulated in a variety of cancers and may contribute to chemoresistance; it also plays a key role in inflammation, especially in inflammatory airway diseases. Roflumilast is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2010 and has 3 approved and 19 investigational indications. After more than two decades of research into phosphodiesterase 4 (PDE4) inhibitors, Roflumilast (3-cyclopropylmethoxy-4-difluoromethoxy-N-[3,5-di-chloropyrid-4-yl]-benzamide) may become the first agent in this class to be approved for patient treatment worldwide. Within the PDE family of 11 known isoenzymes, roflumilast is selective for PDE4, showing balanced selectivity for subtypes A–D, and is of high subnanomolar potency. The active principle of roflumilast in man is its dichloropyridyl N-oxide metabolite, which has similar potency as a PDE4 inhibitor as the parent compound. The long half-life and high potency of this metabolite allows for once-daily, oral administration of a single, 500-μg tablet of roflumilast. The molecular mode of action of roflumilast – PDE4 inhibition and subsequent enhancement of cAMP levels – is well established. To further understand its functional mode of action in chronic obstructive pulmonary disease (COPD), for which roflumilast is being developed, a series of in vitro and in vivo preclinical studies has been performed. COPD is a progressive, devastating condition of the lung associated with an abnormal inflammatory response to noxious particles and gases, particularly tobacco smoke. In addition, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), significant extrapulmonary effects, including comorbidities, may add to the severity of the disease in individual patients, and which may be addressed preferentially by orally administered remedies. COPD shows an increasing prevalence and mortality, and its treatment remains a high, unmet medical need. In vivo, Roflumilast mitigates key COPD-related disease mechanisms such as tobacco smoke-induced lung inflammation, mucociliary malfunction, lung fibrotic and emphysematous remodelling, oxidative stress, pulmonary vascular remodelling and pulmonary hypertension. In vitro, roflumilast N-oxide has been demonstrated to affect the functions of many cell types, including neutrophils, monocytes/macrophages, CD4+ and CD8+ T-cells, endothelial cells, epithelial cells, smooth muscle cells and fibroblasts. These cellular effects are thought to be responsible for the beneficial effects of roflumilast on the disease mechanisms of COPD, which translate into reduced exacerbations and improved lung function. As a multicomponent disease, COPD requires a broad therapeutic approach that might be achieved by PDE4 inhibition. However, as a PDE4 inhibitor, Roflumilast is not a direct bronchodilator. In summary, roflumilast may be the first-in-class PDE4 inhibitor for COPD therapy. In addition to being a non-steroid, anti-inflammatory drug designed to target pulmonary inflammation, the preclinical pharmacology described in this review points to a broad functional mode of action of roflumilast that putatively addresses additional COPD mechanisms. This enables roflumilast to offer effective, oral maintenance treatment for COPD, with an acceptable tolerability profile and the potential to favourably affect the extrapulmonary effects of the disease. [1] Phosphodiesterase 4 (PDE4) is a member of the PDE enzyme superfamily that inactivates cyclic adenosine monophosphate and cyclic guanosine monophosphate, and is the main PDE isoenzyme occurring in cells involved in inflammatory airway disease such as chronic obstructive pulmonary disease (COPD). COPD is a preventable and treatable disease and is characterized by airflow obstruction that is not fully reversible. Chronic progressive symptoms, particularly dyspnoea, chronic bronchitis and impaired overall health are worse in those who have frequent, acute episodes of symptom exacerbation. Although several experimental PDE4 inhibitors are in clinical development, Roflumilast, a highly selective PDE4 inhibitor, is the first in its class to be licensed, and has recently been approved in several countries for oral, once-daily treatment of severe COPD. Clinical trials have demonstrated that roflumilast improves lung function and reduces exacerbation frequency in COPD. Furthermore, its unique mode of action may offer the potential to target the inflammatory processes underlying COPD. Roflumilast is effective when used concomitantly with all forms of bronchodilator and even in patients treated with inhaled corticosteroids. Roflumilast thus represents an important addition to current therapeutic options for COPD patients with chronic bronchitis, including those who remain symptomatic despite treatment. This article reviews the current status of PDE4 inhibitors, focusing on the pharmacokinetics, efficacy and safety of roflumilast. In particular, it provides an overview of the effects of roflumilast on lung function and exacerbations, glucose homoeostasis and weight loss, and the concomitant use of long-acting beta(2)-adrenergic receptor agonists and short-acting muscarinic receptor antagonists. [2] Mechanisms driving persistent airway inflammation in chronic obstructive pulmonary disease (COPD) are incompletely understood. As secretory immunoglobulin A (SIgA) deficiency in small airways has been reported in COPD patients, we hypothesized that immunobarrier dysfunction resulting from reduced SIgA contributes to chronic airway inflammation and disease progression. Here we show that polymeric immunoglobulin receptor-deficient (pIgR(-/-)) mice, which lack SIgA, spontaneously develop COPD-like pathology as they age. Progressive airway wall remodelling and emphysema in pIgR(-/-) mice are associated with an altered lung microbiome, bacterial invasion of the airway epithelium, NF-κB activation, leukocyte infiltration and increased expression of matrix metalloproteinase-12 and neutrophil elastase. Re-derivation of pIgR(-/-) mice in germ-free conditions or treatment with the anti-inflammatory phosphodiesterase-4 inhibitor Roflumilast prevents COPD-like lung inflammation and remodelling. These findings show that pIgR/SIgA deficiency in the airways leads to persistent activation of innate immune responses to resident lung microbiota, driving progressive small airway remodelling and emphysema. [4] Purpose To prove that phosphodiesterase type-4 inhibitors could potentially treat obesity-associated overactive bladder through modulation of the systemic inflammatory response. Methods In this 12-week study, 90 female Sprague–Dawley rats were divided into three groups: (1) vehicle-treated normal diet (ND)-fed rats; (2) vehicle-treated high-fat diet (HFD)-fed rats; and (3) Roflumilast-treated HFD-fed rats. Oral roflumilast (5 mg/kg/day) was administered during the last 4 weeks of HFD feeding in the test group. At 12 weeks, a urodynamic study was performed in ten rats of each group. Bladder tissue was extracted, the bladder mucosa was separated under microscopy, and bladder detrusor smooth muscle (DSM) expression of TNF-α, interleukin (IL)-6, IL-1β, and nuclear factor kappa B (NF-κB) were analyzed using Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Results Bodyweights of the HFD-fed rats significantly increased and were not ameliorated by Roflumilast treatment. Cystometry evidenced augmented frequency and non-void contractions in obese rats that were also prevented by roflumilast. These alterations were accompanied by a markedly increased expression of TNF-α, IL-6, IL-1β, and NF-κB in DSM of obese rats. Furthermore, roflumilast decreased expression of inflammatory factors in DSM. Conclusions Oral treatment with Roflumilast in rats fed an HFD restores normal bladder function and downregulates expression of inflammatory factors in the bladder.[5] Cisplatin (CIS)-induced testicular injury is a major obstacle in its application as antineoplastic agent. In this study, we investigated the protective effect and mechanism of Roflumilast (ROF), a PDE4 inhibitor, against CIS-induced testicular toxicity in rats. Besides, the cytotoxic effect of CIS, with and without ROF, was evaluated on PC3 cell line. ROF reversed CIS-induced abnormalities in sperm characteristics, normalized serum testosterone level, and ameliorated CIS-induced alterations in testicular and epidydimal weights and restored normal testicular structure. Moreover, ROF increased intracellular cAMP level, PKA and HO-1 activities and Nrf2, NQO-1 and HO-1 gene expression, improved testicular oxidative stress parameters (TBARS, NO, GSH levels, and CAT activity) and inflammatory mediators (IL-1β and TNF-α, and NF-κβ p65gene expression) and reduced the proapoptotic proteins, caspase-3, Bax and increased Bcl-2. Lastly, in vitro analyses showed that ROF augmented the anticancer efficacy of CIS and enhanced the increase in gene expression of Nrf2, HO-1, and NQO-1 and the inhibition of gene expression of NF-κβ p65 induced by CIS and enhanced its apoptotic effect in PC3 cells. Conclusively, PDE4 inhibition with induction of Nrf2/HO-1, NQO-1 is a potential therapeutic approach to protect male reproductive system from the detrimental effects with augmenting, the antineoplastic effect of CIS. [6] The results of the current study provide evidence that ROF as a PDE4 inhibitor has a protective effect against CIS-induced male reproductive toxicity. In addition, it provides evidence that inhibition of PDE4 by ROF stimulates the cAMP/Nrf2/HO-1, NQO-1 signaling cascade, which plays a vital role in mitigating the oxidative damage and inflammatory response and attenuated testicular injury induced by CIS in rats. In addition, ROF alone or in combination with CIS triggers prostatic cancer PC3 cell apoptosis by increasing protein and gene expressions of caspase-3, Bax, and Bax/Bcl-2 ratio and decreasing both protein and gene expression of Bcl-2, the effect that can be attributed to stimulation of Nrf2/HO-1 expression and down-regulation of NF-κβ p65 gene expression. Hence, the current study provides a new promising strategy that augments the anticancer effect of CIS and further alleviates its testicular toxicity by its combination with ROF. Further studies in the future are needed to further evaluate the possible clinical application of this combination in cancer chemotherapy.[6] |
| 分子式 |
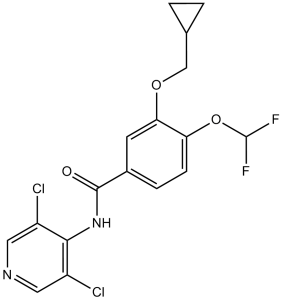
C17H14CL2F2N2O3
|
|---|---|
| 分子量 |
403.2075
|
| 精确质量 |
402.034
|
| 元素分析 |
C, 50.64; H, 3.50; Cl, 17.58; F, 9.42; N, 6.95; O, 11.90
|
| CAS号 |
162401-32-3
|
| 相关CAS号 |
Roflumilast N-oxide;292135-78-5;Roflumilast-d4 N-Oxide;1794760-31-8;Roflumilast Impurity E;1391052-76-8;Roflumilast-d4;1398065-69-4;Roflumilast-d3;1189992-00-4
|
| PubChem CID |
449193
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
430.6±45.0 °C at 760 mmHg
|
| 熔点 |
158°C
|
| 闪点 |
214.2±28.7 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.604
|
| LogP |
4.84
|
| tPSA |
60.45
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
475
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1CC1COC2=C(C=CC(=C2)C(=O)NC3=C(C=NC=C3Cl)Cl)OC(F)F
|
| InChi Key |
MNDBXUUTURYVHR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24)
|
| 化学名 |
3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide
|
| 别名 |
Daliresp; BY217; BY-217; Roflumilast; 162401-32-3; DAXAS; 3-(CYCLOPROPYLMETHOXY)-N-(3,5-DICHLOROPYRIDIN-4-YL)-4-(DIFLUOROMETHOXY)BENZAMIDE; Daliresp; BYK20869; Benzamide, 3-(cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)-; B 9302-107;BYK 20869;B-9302-107;APTA 2217, B9302-107, BY 217, BYK-20869; BYK20869; Daxas;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.20 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol:30 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4801 mL | 12.4005 mL | 24.8010 mL | |
| 5 mM | 0.4960 mL | 2.4801 mL | 4.9602 mL | |
| 10 mM | 0.2480 mL | 1.2400 mL | 2.4801 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05684744 | Completed | Drug: Roflumilast Drug: Methotrexate |
Psoriasis | Cairo University | January 9, 2023 | Phase 2 Phase 3 |
| NCT04322929 | Recruiting | Drug: Roflumilast Oral Tablet | Non-cystic Fibrosis Bronchiectasis | The University of Hong Kong | November 12, 2020 | Phase 2 |
| NCT04549870 | Completed | Drug: Roflumilast | Psoriasis | Bispebjerg Hospital | January 1, 2021 | Phase 2 |
| NCT04108377 | Terminated | Drug: Roflumilast Drug: Placebo |
Asthma | University of California, Davis | January 21, 2019 | Phase 1 |