| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
PDE4
|
|---|---|
| 体外研究 (In Vitro) |
在体外,香烟烟雾提取物 (CSE) 诱导的 WD-HBEC 中的上皮间质转化 (EMT) 被浓度为 2 nM 的罗氟司特 N-氧化物部分抑制。 CSE 后,罗氟司特 N-氧化物 (2 nM) 可以恢复 45% 减少的 E-钙粘蛋白转录物表达。罗氟司特 N-氧化物 (2 nM) 可消除 I 型胶原蛋白的表达。当向细胞中添加罗氟司特 N-氧化物 (2 nM) 时,似乎对上皮细胞表型有保护作用。此外,与罗氟司特 N-氧化物 (2 nM) 预孵育可部分减弱 β-连环蛋白核转位 [2]。
中性粒细胞趋化参与慢性阻塞性肺疾病(COPD)等肺部炎症过程。中性粒细胞弹性蛋白酶(NE)作为中性粒细胞产生的主要蛋白酶之一,通过气道上皮细胞释放趋化因子在炎症过程中起重要作用。近期研究表明罗氟司特N-氧化物对COPD具有治疗潜力。本研究旨在探讨罗氟司特N-氧化物对NE诱导的A549上皮细胞趋化因子产生及信号通路的影响。A549细胞与NE孵育30分钟后PBS洗涤,分别培养2小时(检测mRNA表达)和24小时(检测趋化因子释放)或5-30分钟(检测蛋白磷酸化)。在NE刺激前,细胞单独或联合使用前列腺素E2(PGE2)与罗氟司特N-氧化物进行预孵育。NE刺激可升高A549细胞趋化因子产量并激活p38α通路。而罗氟司特N-氧化物与PGE2联用时,对NE诱导的趋化因子释放及p38α等激酶激活具有协同抑制作用。结论:NE通过激活p38α MAP激酶促进上皮细胞趋化因子释放,而罗氟司特N-氧化物与PGE2联用可降低NE诱导的激酶活化及趋化因子产生。[1] 罗氟司特N-氧化物/RNO与PGE2联用对NE诱导A549上皮细胞趋化因子释放的影响[1]c 单独使用罗氟司特N-氧化物对NE诱导的趋化因子释放无影响(数据未显示)。而10 nM PGE2与RNO联用可降低IL-8/CXCL8、MCP1/CCL2和Gro-α/CXCL1的释放。单独PGE2孵育上皮细胞即可减少NE诱导的上述趋化因子释放(图4A、B、C),但RNO与10 nM PGE2联用能进一步降低MCP1/CCL2和Gro-α/CXCL1的释放(图4B、C)。 NE诱导的p38α激酶通路激活在罗氟司特N-氧化物/RNO处理后减弱[1]v 10 nM PGE2(无论单独或联合RNO)处理上皮细胞5分钟,均显著降低NE诱导的p38α激酶激活(图6A)。但仅RNO与PGE2联用组p38α活化程度显著更低(图6A),该结果经磷酸化p38α特异性ELISA验证(图6B)。PGE2单独或联合RNO均未改变ERK 1/2和JNK通路激活或蛋白总量。 罗氟司特N-氧化物/RNO对NE诱导A549细胞蛋白磷酸化的影响[1]v 首先采用人类磷酸化蛋白阵列试剂盒检测PGE2预处理的A549细胞中10 nM NE是否激活其他信号通路,继而分析RNO与PGE2联用相对于单独PGE2的效果。PGE2预处理后,NE刺激可激活45种激酶中的12种(灰色柱;包括RSK 1/2/3、c-Jun等,激活定义为较对照增加≥30%)(图7A)。当1 μM RNO与PGE2联用时,这12种激酶中有5种(白色柱;包括Hck、FAK等)的激活强度显著降低(图7A、B)。 背景:吸烟通过促进COPD小支气管上皮-间质转化(EMT)参与肺重塑过程。我们近期发现PDE4抑制剂罗氟司特的活性代谢物罗氟司特N-氧化物(RNO)可预防香烟烟雾提取物(CSE)诱导的人支气管上皮细胞EMT。而他汀类药物对肾和肺泡上皮细胞EMT具有保护作用。目的:探讨RNO与辛伐他汀(SIM)对体外培养的小支气管来源高分化人支气管上皮细胞(WD-HBEC)中CSE诱导EMT的交互作用。方法:用2.5% CSE刺激WD-HBEC,通过实时定量PCR或Western blot检测间质标志物(波形蛋白、I型胶原等)、上皮标志物(E-钙黏蛋白等)及β-连环蛋白表达。H2DCF-DA探针检测活性氧(ROS),Western blot分析GTP-Rac1和pAkt。结果:2 nM RNO与100 nM SIM联用对逆转CSE诱导EMT具有(超)叠加效应。CSE通过ROS生成和PI3K/Akt/β-catenin通路激活部分介导EMT。2 nM RNO和100 nM SIM均可部分阻断该通路,两者联用几乎完全抑制ROS/PI3K/Akt/β-catenin信号传导从而阻止EMT。结论:PDE4抑制剂罗氟司特N-氧化物与辛伐他汀在体外WD-HBEC模型中(超)叠加抑制CSE诱导的EMT。[2] |
| 体内研究 (In Vivo) |
在用 10 mg/kg 罗氟司特 N-氧化物处理一次的 db/db 小鼠中,血浆胰高血糖素样肽-1 (GLP-1) 增加了四倍。研究发现,长期服用 3 mg/kg 剂量的罗氟司特 N-氧化物可以阻止 db/db 小鼠的疾病进展。当罗氟司特-N-氧化物作为载体时,它保留了胰岛形状并降低了血糖,HbA1c 分别增加了 50% 和 50%。它还使空腹血清胰岛素增加了一倍。此外,在初级胰岛中,罗氟司特-N-氧化物改善了毛喉素诱导的胰岛素释放。此外,与其母体分子相比,罗罗司特-N-氧化物具有更强的降血糖作用[3]。
单次给予db/db小鼠10 mg/kg罗氟司特或其代谢物罗氟司特N-氧化物,分别使血浆GLP-1水平升高2.5倍和4倍。长期使用3 mg/kg剂量的罗氟司特或罗氟司特N-氧化物可延缓疾病进展。与溶媒组相比,罗氟司特N-氧化物完全阻断了血糖升高,使HbA(1c)增幅降低50%,空腹血清胰岛素翻倍,同时保护胰岛形态。此外,在原代胰岛中该代谢物能增强福司柯林诱导的胰岛素分泌。罗氟司特N-氧化物降糖效果优于母体化合物,与其更强效的GLP-1分泌促进作用一致,药代/药效学模型可解释此现象。 结论:罗氟司特及其活性代谢物罗氟司特N-氧化物可能通过GLP-1和胰岛素相关机制保护胰岛生理功能,从而延缓db/db小鼠糖尿病进展。[3] 单次给药罗氟司特及罗氟司特N-氧化物对血浆GLP-1的影响[3] 在空腹db/db小鼠中观察10 mg/kg单剂量罗氟司特或其代谢物对血浆GLP-1的作用,并评估葡萄糖刺激(GLP-1分泌生理触发剂)的调节效应。葡萄糖存在时,罗氟司特和罗氟司特N-氧化物分别使血浆GLP-1较溶媒组显著增加2.5倍和4.3倍(图1)。该效应在给药10分钟后显现,60分钟后消退。无葡萄糖刺激时,两者仅引起GLP-1轻微非显著性升高(数据未显示)。 长期给药罗氟司特及罗氟司特N-氧化物对体重与摄食饮水的影响[3] 3 mg/kg/天剂量的罗氟司特及其代谢物显著降低db/db小鼠摄食量与饮水量(图2a,b)。治疗4周后平均摄食量较溶媒组减少约17%。溶媒组动物饮水量从6.7 g/天增至15.6 g/天,而罗氟司特与罗氟司特N-氧化物治疗组分别降至7.6 g/天和3.8 g/天。此效应在首次给药后即出现并持续整个研究周期。两者对体重无显著影响(图2c)。 长期给药罗氟司特及罗氟司特N-氧化物对血液指标的影响[3] 溶媒组动物HbA1c在4周内从4.4%(24.6 mmol/mol)升至8.2%(66.1 mmol/mol)。3 mg/kg/天治疗显著抑制HbA1c升高,罗氟司特N-氧化物治疗4周后增幅较溶媒组降低50%(p<0.01)(图3a)。与之对应,溶媒组血糖AUC−15–60和空腹血糖分别升高38%和51%,而罗氟司特N-氧化物使两者恢复至研究初期水平(分别p<0.001和p<0.01),罗氟司特仅显著降低血糖AUC(p<0.01)(图3b-d)。溶媒组空腹血清胰岛素从602 pmol/l轻微下降至551 pmol/l,而罗氟司特N-氧化物治疗4周后该指标近乎翻倍(p<0.05)。葡萄糖刺激后血清胰岛素水平在两组均未升高(数据未显示)。 长期给药罗氟司特及罗氟司特N-氧化物对胰腺形态的影响[3] H&E染色显示11周龄溶媒组db/db小鼠出现轻度至中度胰岛萎缩(25-50%胰岛受累,萎缩严重度评分2.6)(图4)。连续切片胰岛素染色证实萎缩胰岛伴随β细胞减少(图5a,b)。每日3 mg/kg治疗4周后,罗氟司特组胰岛萎缩程度减轻(评分2.0,p>0.05),罗氟司特N-氧化物组萎缩极轻微(评分1.3,p<0.01),且两组均保留β细胞胰岛素分泌功能(图5c,d)。 罗氟司特N-氧化物增强小鼠胰岛胰岛素分泌[3] 在原代小鼠胰岛中验证该代谢物对胰岛素分泌的促进作用(图7)。腺苷酸环化酶广谱激活剂福司柯林(1 μmol/l)使胰岛素分泌增加4倍,当联合10或100 nmol/l罗氟司特N-氧化物时,10 nmol/l葡萄糖条件下的胰岛素释放分别增强至5.5倍和7倍。单独使用罗氟司特(数据未显示)或其代谢物在不同葡萄糖浓度下均不能诱导胰岛素分泌(图示为100 nmol/l代谢物在10 mmol/l葡萄糖条件下的结果)。 |
| 细胞实验 |
治疗[1]
A549细胞在添加抗生素、l-谷氨酰胺和HEPES的无血清f - 12k培养基中洗涤培养过夜。将饥饿后的细胞用NE或PBS孵育30分钟,用PBS洗涤,然后在无血清的f - 12k中培养。刺激后24 h收集细胞上清液(用于细胞因子测量),2 h收集细胞微球(用于mRNA表达分析)。另外,在加入NE之前,A549细胞与PGE2 (10 nM)单独或与Roflumilast N-oxide (0.1 μM, 0.3 μM和1 μM),载体(DMSO 0.01%)或EGFR抑制剂AG-1478联合预孵育2小时。所有实验均在无血清培养基中进行,一式三份,至少重复三次。在孵育期结束时,收集培养上清液并在- 80°C保存,以待进一步分析。 人磷蛋白阵列[1] 在加入NE之前,将细胞与PGE2 (10 nM)单独或与Roflumilast N-oxide (1 μM)联合预孵育2 h。根据制造商的说明,将细胞裂解液(每个阵列总蛋白500 μg)应用于磷酸化蛋白阵列(Proteome Profiler Human Phosphokinase array Kit)。 |
| 动物实验 |
Acute studies in db/db mice [3]
At 7 weeks of age, 16 h fasting mice received a single oral dose of vehicle (4% methocel) or test compound (10 mg/kg roflumilast or Roflumilast N-oxide, maximal pharmacological effective dose without side effects following single administration), and a glucose bolus of 2 g/kg body weight was co-administered as a physiological initiator for GLP-1 secretion. The glucose concentration of 2 g/kg body weight was identified in pre-experiments as being optimal because it did not markedly induce GLP-1 levels on its own but potently triggered GLP-1 release in concert with our PDE4 inhibitors. Plasma GLP-1 was analysed 60 min before, and 10 and 60 min after administration of PDE4 inhibitor and glucose. The effect of roflumilast and Roflumilast N-oxide on plasma GLP-1 was also investigated in the absence of the glucose bolus. Chronic study in db/db mice [3] At 7 weeks of age, all animals were treated once daily by oral gavage with vehicle (4% methocel), roflumilast or Roflumilast N-oxide. Body weight and food and water intake were monitored daily. Animals destined for pharmacodynamic analysis (PD animals) were treated with roflumilast (0.3, 1 and 3 mg kg–1 day–1) or Roflumilast N-oxide (3 mg kg–1 day–1) for 28 days, with 3 mg/kg being the maximal pharmacological effective dose without side effects following chronic administration. HbA1c was determined 6 days and 1 day before, and 14 and 28 days after treatment start. At days 1 and 28, an OGTT was performed for analysis of blood glucose and serum insulin. For OGTT, animals received a glucose bolus of 1 g/kg body weight after fasting for 4 h. In contrast to 16 h fasting in the acute study, the shorter 4 h fasting period results from method optimisation experiments we performed for ethical reasons and provides comparable results to those with 16 h fasting. Blood was collected 15 min before and 15 and 60 min after the glucose challenge. The glucose AUC was determined from time −15 to 60 min. Additional blood samples were taken at days 20 and 28 after treatment start for drug exposure analysis. Animals were killed by cervical dislocation on day 28 and the pancreas was removed for histopathological examination. For pharmacokinetic (PK) analysis, we included satellite animals (PK animals) which were treated with 3 mg kg–1 day–1 roflumilast or Roflumilast N-oxide for 34 days. At days 1, 14, 20, 34 and 35, blood samples were taken for drug exposure analysis. PKPD analysis in chronic db/db mouse study [3] Drug exposure was analysed in blood samples from PD and PK animals taken at different time points, with each animal providing two and eight samples, respectively (PD animals: −0.5/2 h, day 20; 30 h, day 28. PK animals: 0.5/1/2/4/8 h, day 1; 0.5/1/4 h, day 14; 0.5/2 h, day 20; −0.5/0.5/1/2/4/8 h, day 34; 30 h, day 35). Samples with values greater than the lower limit of quantification (LLOQ) were used for analysis (HPLC-MS/MS, LLOQ = 0.5 μg/l) after Roflumilast N-oxide dosing, corresponding to 51 and 60 concentration values being available for parent and metabolite, respectively. After roflumilast dosing, 89 concentration values were available for both parent and metabolite, and values below LLOQ were set to half LLOQ (0.25 μg/l) to reduce model bias. Four PK models were developed, that is for both roflumilast and its metabolite after both roflumilast and Roflumilast N-oxide dosing, respectively. All variables were estimated using the nonlinear mixed-effects modelling (NONMEM) technique (version 7, ICON Development Solutions, Ellicott City, MD, USA) with first-order conditional estimation with interaction. From the obtained individual apparent clearance estimates, individual steady state AUC was calculated for each PD animal according to the following equation: AUC (μg h l−1) = dose (μg/kg)/clearance (l h−1 kg−1). AUC estimates were used to determine total PDE4 inhibition (tPDE4i) of the respective compounds for all PD animals. The tPDE4i relates the average free concentration of a compound in plasma to its in vitro IC50 of PDE4 inhibition, and is an exposure surrogate allowing for the consideration of parallel contribution of parent and metabolite to the overall effect. [3] with rof and rofNO corresponding to roflumilast and Roflumilast N-oxide, fu corresponding to the unbound fraction in mouse plasma in vitro, IC50 corresponding to the compound concentration resulting in 50% PDE4 inhibition in vitro and τ corresponding to the dosing interval. To consider the presence of both roflumilast and roflumilast-N-oxide in the circulation, tPDE4i values calculated separately for each compound were added. The combined individual tPDE4i values were used as a measure of exposure in the subsequent PKPD model. HbA1c levels on days −1, 14 and 28 from vehicle, roflumilast and roflumilast-N-oxide groups were used as PD readout, and were related to individual tPDE4i values using the following equation: |
| 参考文献 |
|
| 其他信息 |
We found that NE is able to increase the release of chemokines from epithelial cells via the activation of p38α MAP-kinase. Moreover, we showed that an EGF receptor inhibitor is able to inhibit NE-induced production of IL-8/CXCL8 and Gro-α/CXCL1 but not of MCP-1/CCL2. In contrast, treatment of the cells with a combination of RNO and PGE2 was associated with lower IL-8/CXCL8, MCP-1/CCl2 and Gro-α/CXCL1 release and a lower degree of activation of several kinases. Our results confirm RNO's anti-inflammatory effect in this in vitro elastolytic model and help to explain the compound's mechanism of action.[1]
Roflumilast N-oxide and simvastatin may prevent the CSE-induced increase of GTP-bound Rac1 by complementary mechanisms resulting in an additive interaction. While the statin limits geranylgeranylation hence, Rac1 membrane docking the PDE4 inhibitor would curb the GDP→GTP exchange. Given the critical role of GTP-Rac1 for NOX1/NOX2 activity the additive effects of roflumilast N-oxide and simvastatin to suppress CSE-induced ROS may reflect their inhibition of GTP-Rac1. As previously shown removal of ROS protects from CSE-induced EMT in WD-HBEC (Citation4). Therefore, that roflumilast N-oxide and simvastatin were additive to prevent CSE-induced EMT could be attributed to their suppression of CSE-induced ROS in WD-HBEC. That simvastatin and Roflumilast N-oxide prevented CSE-induced activation of the PI3K/Akt pathway and the ensuing increase in nuclear β-catenin in WD-HBEC is a novel finding. One plausible explanation however, may be their ability to attenuate CSE-induced ROS in WD-HBEC. In conclusion the current work shows additive effects of the PDE4 inhibitor Roflumilast N-oxide and the HMG-CoA reductase inhibitor simvastatin to mitigate EMT secondary to tobacco smoke in well-differentiated human bronchial epithelial cells. The PDE4 inhibitor and the statin may act on different pathways involved in CSE-induced EMT reflected by inhibition of GTP-Rac1, ROS, PI3K/Akt and nuclear β-catenin. [2] To our knowledge, we have shown for the first time the effect of PDE4 inhibition in an animal model of type 2 diabetes evaluating disease progression in detail and analysing the effects on GLP-1 and insulin being central hormones in glucose metabolism. Roflumilast was used as a selective PDE4 inhibitor, previously approved as a drug for the treatment of COPD. A recent human study with roflumilast addressed the question of efficacy in type 2 diabetes (E.F.M Wouters, Maastricht University Medical Center, Maastricht, the Netherlands, unpublished results). In humans, roflumilast is metabolised to the active metabolite Roflumilast N-oxide, which exhibits a nearly 10-fold higher exposure compared with its parent compound and acts as the principal contributor to the roflumilast drug effect. As roflumilast metabolism in db/db mice was unknown, we included one treatment arm with roflumilast-N-oxide to assure efficacious plasma concentrations. In accordance with the previously reported GLP-1 elevating effect of rolipram in non-diabetic rats, we now confirm the result in a type 2 diabetes model. In diabetic db/db mice, single oral administration of 10 mg/kg roflumilast or its metabolite increased plasma GLP-1 vs vehicle in the presence of glucose by a factor of 2.5 and 4.3, respectively. As this report showed in addition that rolipram is able to directly stimulate the cAMP-mediated GLP-1 release in GLUTag cells, we concluded that the observed increase of GLP-1 in response to roflumilast results from cAMP-mediated GLP-1 release in intestinal L-cells of the db/db mice. In the absence of glucose, roflumilast and Roflumilast N-oxide had no significant effect on plasma GLP-1 suggesting that PDE4 inhibition amplifies GLP-1 release only following food intake, which is the physiological initiator of GLP-1 secretion. In contrast, therapeutic GLP-1 mimetics elevate GLP-1 levels independent of food intake. Chronic treatment of db/db mice with roflumilast or Roflumilast N-oxide clearly ameliorated the diabetic status of the animals. Roflumilast-N-oxide at 3 mg kg–1 day–1 almost abolished the increase in glucose AUC and fasting glucose over the study time and reduced the increment in HbA1c by about 50% vs vehicle. In addition, fasted serum insulin levels almost doubled following 4 weeks of treatment concomitant with amelioration of pancreatic islet morphology and preservation of insulin production in beta cells. The improved diabetic status was also reflected by a reduction in water and, although less pronounced, food consumption relative to control. Water consumption decreased to 3.8 g/day, a level we observed in healthy mice (data not shown), suggesting that the renal glucose reabsorption capacity was no longer overloaded in contrast to vehicle-treated mice showing a high water intake of 15.6 g/day at the end of the study. Despite its food-reducing effect, roflumilast-N-oxide had no effect on body weight development. However, this discrepancy might be a db/db mouse-specific effect as PDE4 inhibitors have been shown to reduce body weight in diet-induced obesity mice and diabetic COPD patients. This indicates that PDE4 inhibitors might be advantageous over current glucose-lowering drugs such as insulin, sulfonylureas or thiazolidinones, for which body weight gain is often an adverse event. Compared with its parent roflumilast, chronic Roflumilast N-oxide treatment resulted in stronger glucose-lowering effects as demonstrated by a greater reduction of blood glucose and HbA1c and superior islet preservation. The effect on food intake was similar for both compounds; however, this may be attributed to the limited sensitivity of the method. The stronger glucose-lowering effect of roflumilast-N-oxide following chronic treatment is consistent with its greater GLP-1-increasing effect following acute treatment. This observation and extensive published literature regarding the glucose-lowering role of GLP-1 support the view that roflumilast-mediated GLP-1 elevation and the prevention of diabetes progression in our db/db mice are linked. In particular, treatment of db/db mice with GLP-1 mimetics shows parallels to our study results with respect to reduced food and water consumption and preservation of pancreatic islets. Another observation was striking to us, supporting our interpretation that PDE4 inhibitors ameliorate diabetes likely via enhancement of physiological intestinal GLP-1 release. Comparing drug reaction profiles of GLP-1 mimetics and PDE4 inhibitors in humans shows that both treatments can reduce body weight and cause gastrointestinal side effects such as delayed gastric emptying, transient nausea and diarrhoea. A reason for the stronger glucose-lowering effect of Roflumilast N-oxide compared with roflumilast is given by PK modelling as shown by a 1.6-fold higher value of the combined exposure surrogate tPDE4i after roflumilast-N-oxide treatment compared with treatment with the parent roflumilast. Furthermore, the modelling results suggest that PDE4 inhibitors carry the potential to keep HbA1c at starting levels in db/db mice based on the estimated EMAX of 1. As the estimated PDE50 of 27.1 in db/db mice is approximately 27-fold higher than the tPDE4i of 1.03 in humans at the effective dose of 500 μg [30], db/db mice seem to require higher exposures for efficacy than humans. This should be considered in the translation of results from mice to humans. [3] |
| 分子式 |
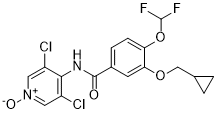
C17H14CL2F2N2O4
|
|---|---|
| 分子量 |
419.2069
|
| 精确质量 |
418.029
|
| 元素分析 |
C, 48.71; H, 3.37; Cl, 16.91; F, 9.06; N, 6.68; O, 15.27
|
| CAS号 |
292135-78-5
|
| 相关CAS号 |
Roflumilast;162401-32-3;Roflumilast Impurity E;1391052-76-8
|
| PubChem CID |
9940999
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
519.7±50.0 °C at 760 mmHg
|
| 熔点 |
181 °C
|
| 闪点 |
268.1±30.1 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.616
|
| LogP |
1.43
|
| tPSA |
73.05
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
645
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1CC1COC2=C(C=CC(=C2)C(=O)N=C3C(=CN(C=C3Cl)O)Cl)OC(F)F
|
| InChi Key |
KHXXMSARUQULRI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H14Cl2F2N2O4/c18-11-6-23(25)7-12(19)15(11)22-16(24)10-3-4-13(27-17(20)21)14(5-10)26-8-9-1-2-9/h3-7,9,17,25H,1-2,8H2
|
| 化学名 |
3-(cyclopropylmethoxy)-N-(3,5-dichloro-1-hydroxypyridin-4-ylidene)-4-(difluoromethoxy)benzamide
|
| 别名 |
roflumilast N-oxide; 292135-78-5; Benzamide, 3-(cyclopropylmethoxy)-N-(3,5-dichloro-1-oxido-4-pyridinyl)-4-(difluoromethoxy)-; F08MQ6CZCS; DTXSID80433059; ROFLUMILAST METABOLITE M07; BYK22890; 3-(Cyclopropylmethoxy)-N-(3,5-dichloro-1-oxido-4-pyridinyl)-4-(difluoromethoxy)benzamide;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~596.36 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.96 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.96 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3854 mL | 11.9272 mL | 23.8544 mL | |
| 5 mM | 0.4771 mL | 2.3854 mL | 4.7709 mL | |
| 10 mM | 0.2385 mL | 1.1927 mL | 2.3854 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。