| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
C3a receptor (IC50 = 200 nM)
|
|---|---|
| 体外研究 (In Vitro) |
SB 290157 的 IC50 为 200 nM,可作为 125I-C3a 放射性配体的竞争性拮抗剂,与表达人 C3aR (RBL-C3aR) 的大鼠嗜碱性白血病-2H3 细胞结合。 SB290157抑制 RBL-C3aR 细胞和人中性粒细胞中 C3a 诱导的 Ca2+ 动员和 C3a 诱导的 C3aR 内化,IC50 值分别为 27.7 和 28 nM。由于 SB 290157 不会与 C5aR 或其他六种趋化性 G 蛋白偶联受体发生激动性相互作用,因此它对 C3aR 表现出选择性。此外,在 C3a 诱导的 Ca2+ 动员方面,表达小鼠和豚鼠 C3aR 的 RBL-2H3 细胞会受到 SB 290157 的抑制。它能有效抑制 C3a 诱导的对灌注大鼠尾动脉场刺激的收缩反应的增强,以及 C3a 介导的豚鼠血小板 ATP 释放[1]。
过敏毒素C3a是一种强效的趋化肽和炎症介质,在补体激活过程中释放,与G蛋白偶联受体结合并激活。C3aR的分子克隆促进了鉴定C3aR非肽拮抗剂的研究。鉴定并化学优化了一种在高通量筛选中选择性抑制C3aR的化学先导物。产生的拮抗剂N(2)-[(2,2-二苯乙氧基)乙酰基]-L-精氨酸(SB290157)作为(125)I-C3a放射性配体与表达人C3aR(RBL-C3aR)的大鼠嗜碱性白血病(RBL)-2H3细胞结合的竞争性拮抗剂,IC(50)为200 nMSB290157是一种功能拮抗剂,以浓度依赖的方式阻断C3a诱导的C3aR内化,并阻断C3a在RBL-C3aR细胞和人中性粒细胞中诱导的Ca(2+)动员,IC(50)分别为27.7和28 nM。SB 290157对C3aR具有选择性,因为它不拮抗C5aR或其他六种趋化性G蛋白偶联受体。功能拮抗不仅限于人类C3aR;SB 290157还抑制了C3a诱导的表达小鼠和豚鼠C3aRS的RBL-2H3细胞的Ca(2+)动员:它能有效抑制C3a介导的豚鼠血小板ATP释放,并抑制C3a诱导对灌注大鼠尾动脉场刺激的收缩反应增强[1]。 为了鉴定非肽C3aR拮抗剂,使用由RBL-C3aR细胞和125I-C3a制备的膜配置了高通量放射配体结合分析。在高通量筛选中测试了来自SmithKline Beecham化合物集合的约240000种化合物,得到64种确认的活性化合物。其中一种化合物SKF 63649作为选择性C3aR拮抗剂得到了进一步的研究进展(图1,a)。随后对该化合物的化学优化导致了SB290157的发现(图1,B)。在125I-C3a竞争性结合实验中评估了这两种化合物对C3aR的亲和力。在该测定中,SB 290157对C3aR的亲和力比SKF 63649高一个数量级;IC50值分别为200和3000 nM(图2,A)。相关结构SB 280936(图1C)在浓度高达10μM的竞争性结合试验中对该受体没有亲和力,并用作阴性对照。 为了确定这些化合物是否是功能性拮抗剂,使用了基于FLIPR的C3a诱导的RBL-C3aR细胞中Ca2+动员试验。SKF 63649和SB290157分别表现出对1 nM C3a诱导的Ca2+动员的浓度依赖性抑制,IC50分别为350 nM(n=2)和27.7±2.9 nM(=3)(图2B)。在高达20μM的浓度下,SB 280936对C3a诱导的RBL-C3aR细胞中Ca2+动员没有影响。通过测试天然表达C3aR的细胞的活性,我们研究了拮抗剂抑制新鲜分离的外周血中性粒细胞中C3a诱导的Ca2+动员的能力。这两种化合物都是拮抗剂,对SKF 63649和SB290157的IC50分别为388和30 nM。SB 290157对C3aR具有选择性,因为它不会拮抗C5a诱导的人中性粒细胞或RBL-C5aR细胞中的Ca2+动员,也不会抑制中性粒细胞上其他五种GPCR的Ca2+调动反应,即白三烯B4、fMLP、血小板活化因子、CXCR1和CXCR2。 对SB290157抑制C3a诱导的HMC-1细胞趋化性的能力进行了评估,HMC-1细胞是一种天然表达C3aR的人肥大细胞系,C3a对其具有趋化性。浓度为5μM的SB 290157显著抑制了C3a介导的HMC-1细胞的趋化性(图2C)。SB 290157对HMC-1细胞的C5a介导的趋化性没有影响(数据未显示)。 测试拮抗剂对C3a诱导的C3aR内化的抑制作用。中性粒细胞与10 nM C3a孵育3分钟足以刺激90%的C3aR内化。SKF 63649和SB290157均以浓度依赖的方式抑制了10 nM C3a诱导的C3aR内化(图3)。在拮抗剂浓度大于1μM的情况下,C3a诱导的C3aR内化减少了约50%(图3)。在该试验中,SB 280936对C3aR内化没有影响(图3)。 除了对人C3aR的功能拮抗作用外,SB290157还是C3a诱导的稳定表达小鼠和豚鼠C3aRs的RBL 2H3细胞Ca2+动员的强效抑制剂(表I)。SB290157抑制C3a诱导的小鼠和豚鼠C3aRs的Ca2+动员的IC50分别为7和12.5 nM。SB 280936在小鼠和豚鼠C3aRs上均无活性。 为了评估拮抗剂对人类以外物种内源性C3aRs的功能活性,评估了它们对1 nM(本试验中的EC80浓度)C3a诱导的豚鼠血小板ATP释放的抑制作用,豚鼠血小板是天然表达C3aR的细胞。SKF 63649和SB290157均以浓度依赖的方式抑制,IC50值分别为385±185和30±14 nM[1]。 |
| 体内研究 (In Vivo) |
在豚鼠 LPS 诱导的气道中性粒细胞增多和大鼠佐剂诱导的关节炎模型中,SB290157分别减轻爪水肿并抑制中性粒细胞募集[1]。只有三小时后,拮抗剂才能有效消除关节肿胀;在 30 mg/kg 剂量下,观察到关节肿胀可抑制 50%。三小时后,C3 水平远低于表现出补体消耗的初始小鼠。此外,值得注意的是,C3 激活随着抗 OVA pAb 的分级浓度而增加 [2]。
在豚鼠LPS诱导的气道中性粒细胞减少模型中评估了SB290157。如图6所示,以气溶胶形式给药的LPS(10μg/ml)在LPS暴露后48小时产生白细胞浸润(比未暴露的动物高5倍),尤其是中性粒细胞(>1000倍)。通过给予SB290157,30mg/kg i.p.b.i.d.,气道中性粒细胞减少(39%)(LPS+载体=33.2±300万中性粒细胞或50.4%的总白细胞恢复;LPS+SB 290157=20.3±170万中性粒血球或31.5%的总白血球;p=0.02,Fisher保护最小二乘差)。治疗动物的白细胞总数与载体治疗动物没有显著差异(LPS+载体=65.1±1130万;LPS+SB 290157=62.0±1010万)。 还使用预防性给药方案在佐剂诱导的关节炎模型中评估了SB290157。从佐剂注射当天开始,将化合物施用于雄性Lewis大鼠。SB 290157经腹膜内给药,每日两次,并在第20天测量爪子炎症。在连续20天接受30mg/kg b.i.d剂量的动物中,第20天爪水肿抑制率为41%(p<0.001)。接受3或10 mg/kg SB 290157 b.i.d.的大鼠足爪水肿没有显著影响[1]。 研究了C3a受体拮抗剂(C3aRA)是否参与抑制抗OVA pAb诱导的关节炎,因为众所周知,过敏毒素C3a在补体激活期间有效炎症反应的发展中起着至关重要的作用。在DBA/1J小鼠中,通过在关节内(i.a.)注射OVA(0小时)前0.5小时给予抗OVA pAb诱导关节炎。在0.5和3小时观察到关节肿胀的两个峰值。通过在0和2小时注射浓度为10和30 mg/kg的SB290157,研究了C3aRA在关节炎中的作用。拮抗剂仅能在3小时减少关节肿胀,30 mg/kg的浓度对关节肿胀的抑制率约为50%。与显示补体消耗的幼稚小鼠相比,C3水平在3小时显著降低。此外,观察到C3活化,并相应于抗OVA pAb的分级浓度而增加。结果还表明,C3aRA能够降低滑膜组织中IL-1β的表达。综上所述,结果表明C3aRA可能有效抑制关节炎[2]。 |
| 酶活实验 |
受体内化试验[1]
如所述,使用C3aR特异性兔多克隆抗血清进行流式细胞术内化测定。测试了100 nM至10μM范围内的SB290157、1-萘氧基乙酰精氨酸(SKF 63649)和N-[(3,5-二氯苯基)甲基]-N-(3-吡啶羰基)甘油-1-精氨酸](SB 280936)在用10 nM C3a刺激人类中性粒细胞后抑制C3aR内化的能力,该浓度诱导C3aR从细胞表面几乎完全消失(>90%的受体内化)。中性粒细胞在37°C下与化合物和10 nM C3a共孵育3分钟,然后评估受体内化情况。 结合分析[1] C3a拮抗剂的高通量闪烁邻近试验(SPA)。[1] 使用RBL-C3aR细胞膜建立了一种主要的高通量放射性配体结合测定法。125I-C3a以高亲和力(Kd=8 pM)结合RBL-C3aR细胞,并且是可饱和的。 所有结合分析均在96孔微量滴定板中进行。Bolton Hunter定制碘化由NEN Research Products使用sp.act进行。2200 Ci/mmol。结合缓冲液由20 mM双三丙烷(pH 8.0)、25 mM NaCl、1 mM MgSO4和0.1 mM EDTA组成。每个孔含有:125I-C3a(16 pM)、70μg麦胚凝集素SPA珠、0.20μg RBL-C3aR膜、23μg/ml BSA和0.03%的3-[(3-甲酰氨基丙基)二甲基氨基]-1-丙磺酸结合缓冲液。此外,非特异性结合的对照孔包括超过15nM的未标记C3a。 在冰上摇动的同时,将膜与SPA珠预结合30分钟。将膜和珠粒的混合物在2000rpm下离心3分钟。去除上清液,将沉淀物重新悬浮在含有50μg/ml BSA的结合缓冲液中至原始体积,然后分配到微量滴定板中。将拮抗剂溶解在纯DMSO中,得到20倍的溶液,然后与H2O按1:1的比例混合,得到10倍、50%的DMSO工作溶液。添加顺序为10μl样品、45μl膜结合SPA珠,然后是45μl放射性标记配体,在含有0.06%3-[(3-氨基丙基)二甲基铵]-1-丙磺酸酯的结合缓冲液中。用Dynex Technologies的板密封剂覆盖板,摇动20分钟,并在室温下再孵育40分钟。然后将平板以2000rpm离心3分钟,然后在Wallac 1450 MicroβPlus液体闪烁计数器上计数。 对后续研究具有约束力。[1] 结合测定基本上如前所述进行(26)。简而言之,在室温下,将2-5×105个RBL-C3aR细胞与100 pM 125I-C3a和不同浓度的拮抗剂在20 mM HEPES(pH 7.4)、125 mM NaCl、5 mM KCl、1 mM CaCl2、1 mM MgCl2、0.25%BSA和0.5 mM葡萄糖(HAG-CM)中孵育45分钟。使用HV Millipore MultiScreen测定板通过真空过滤去除未结合的配体,该板具有用HAG-CM平衡的Durapore 0.45-μm孔径膜。用100μl/孔的HAG-CM洗涤过滤器两次并干燥。在贝克曼伽马计数器5500B上对板进行计数。使用KaleidaGraph v3.09进行数据分析。 |
| 细胞实验 |
HMC-1趋化性测定[1]
使用Neuro Probe 96孔一次性趋化板(5μm孔径)评估HMC-1细胞的C3a介导的趋化性。膜的顶面预先涂有100ng层粘连蛋白或纤维连接蛋白。在28μl RPMI 1640中向较低的孔中加入不同浓度的C3a(有和没有拮抗剂)。组装过滤器,将2至5×105个细胞加入25μl的顶部孔中。将板在37°C和5%CO2下孵育60分钟。移除过滤器,用PBS冲洗膜的顶面;然后用Diff-Quik(Baxter,Dade Division,Miami,FL)对细胞进行染色。通过在三个连续的高功率场中计数细胞,在显微镜下定量迁移的细胞数量。 荧光成像板读数仪(FLIPR)Ca2+动员测定[1] 使用Fluo 3负载的RBL-C3aR细胞和使用FLIPR的微量滴定板进行C3aR Ca2+动员研究。简而言之,收获细胞(约80%融合),以约40000个细胞/孔的速度将其铺在96孔黑壁透明底板(Packard观察板)上,并在培养箱中生长18-24小时。在测定当天,吸出培养基,用100μl Eagle's MEM替换,该MEM含有l-谷氨酰胺、0.1%BSA、4μM氟-3-乙酰氧基甲酯和1.5 mM亚磺吡喃酮的Earle盐。将板在37°C下孵育60分钟;吸出培养基,用不含氟-3-乙酰氧基甲酯的相同培养基替换,并在37°C下孵育10分钟。将细胞洗涤三次,并在37°C下在100μl测定缓冲液(120 mM NaCl、4.6 mM KCl、1.03 mM KH2 PO4、25 mM NaHCO3、1.0 mM CaCl2、1.1 mM MgCl2、11 mM葡萄糖、20 mM HEPES(pH 7.4)和1.5 mM亚磺吡喃酮)中孵育。如前所述,将板放入FLIPR中进行分析。定量了加入激动剂后荧光的最大变化。测定每种拮抗剂浓度下C3a诱导的最大Ca2+动员百分比。IC50定义为抑制1nM C3a诱导的最大反应的50%的测试化合物的浓度,从浓度-反应曲线中获得。对于激动剂效力,EC50定义为产生50%最大C3a诱导反应的浓度。 |
| 动物实验 |
Contraction of rat caudal artery [1]
Male Sprague Dawley normotensive rats weighing between 400 and 600 g were euthanized, and the tail was removed and placed in physiologic buffer. The tail was secured to a dissection board, the caudal artery was exposed, and a 30- to 40-mm-long section of the artery was dissected from the tail and placed into buffer. The artery section was cut into two segments of equal length, each segment was cannulated at both ends with PE50 tubing, and the tubing was secured with ties of 4-0 surgical silk. The cannulated arterial segments were mounted in a tubular glass chamber and were simultaneously perfused intraluminally and superfused extraluminally with oxygenated Krebs buffer at 38°C. The rate of intraluminal perfusion was 1 ml/min, and that of extraluminal superfusion was 2 ml/min. Under these conditions, the baseline perfusion pressure equilibrated to between 25 and 50 mm Hg. After a 20- to 30-min stabilization period, the periarterial sympathetic nerves were stimulated electrically every 30 s via platinum electrodes located at both ends of the chamber to obtain a brief, spike-like increase in perfusion pressure. The stimulation consisted of a 1-s train of square wave pulses at 70 V of 0.7 ms duration and a frequency of 15 Hz. These stimulation parameters resulted in a 50- to 100-mm Hg increase in perfusion pressure above baseline. When the response stabilized, one of the arterial segments was exposed to SB290157 delivered in the superfusion flow, and the other artery was left untreated. After a 15-min exposure to SB290157 (10 nM, 100 nM, and 1 μM), C3a (100 nM) was introduced in the superfusion flow to both arterial segments, and the effect on perfusion pressure was monitored. Typically, C3a enhanced the perfusion pressure. The C3a-mediated increase in perfusion pressure was rapidly desensitized (1–2 min). Guinea pig airway neutrophilia model [1] Male Hartley guinea pigs were obtained from Charles River Breeding Laboratories and maintained in a barrier facility. Guinea pigs were placed four at a time into a plastic box (20 liters) that had been modified with an intake and exhaust port; a small fan in the lid increased aerosol circulation. An LPS aerosol dissolved in normal saline (30 μg/ml) was generated by a modified DeVilbiss Pulmosonic nebulizer and delivered for 15 min into the box via the intake port at a rate of 250 ml/min. SB290157 (30 mg/kg) or vehicle (20% polyethylene glycol 400 (PEG) in saline) was administered i.p. 1 h before and 4 h after LPS challenge and administered twice a day (b.i.d.) 6 h apart on the next day. A third group of animals were left unexposed to LPS and received vehicle alone. Bronchoalveolar lavages (BAL) were performed 48 h after LPS exposure. Guinea pigs were euthanized by pentobarbital overdose, and the lungs were lavaged with 50 ml Dulbecco’s PBS (5 × 10 ml), which was aspirated after a gentle chest massage. The BAL fluid was centrifuged, and the pellet was resuspended in 0.25% NaCl to lyse residual erythrocytes; after centrifugation, the pellet was resuspended again in 1 ml 0.9% NaCl. After total cells were counted, slides were prepared, stained, and differentiated as eosinophils, neutrophils, and mononuclear cells by counting a minimum of 200 cells and expressing the results as percentage of total cells as well as actual numbers of each type. This measurement and expression technique has been previously validated, by histological methods, as accurately reflecting endothelial and subendothelial airway leukocytosis. Cell number and percentages were statistically compared by ANOVA followed by Fisher’s protected least square difference test. Adjuvant-induced arthritis [1] Male inbred Lewis rats were obtained from Charles River Breeding Laboratories. Within a given experiment, only animals of the same age were used. Adjuvant-induced arthritis (AIA) was induced as described previously. Briefly, 0.75 mg of Mycobacterium butyricum suspended in paraffin oil was injected into the base of the tail of male Lewis rats 6–8 wk old (160–180 g). Hind paw volumes were measured by a water displacement method on day 20. SB290157 was suspended in a vehicle consisting of 5% ethanol, 10% Cremaphor-El, and 85% saline and administered b.i.d. at 30, 10, and 3 mg/kg i.p. in a final volume of 0.5 ml starting on the day of adjuvant injection. Cages were modified to allow the compromised animals free access to food and water. Control animals were given vehicle alone. Change in paw volume is presented as mean and SEM of 10–12 animals/group, and the percentage inhibition of hind paw edema was calculated as described. For statistical analysis, paw volumes of rats treated with SB290157 were compared with the untreated controls by Student’s t test. Pharmacokinetic studies in guinea pigs [1] A pharmacokinetic study was conducted using three male Hartley guinea pigs. Under aseptic conditions, each guinea pig received surgically implanted femoral and arterial vein catheters at least 5 days before the study day. On the study day, fed animals received SB290157 (30 mg/kg) as a single i.p. bolus injection (3 ml/kg total volume). The dose solution was prepared in normal saline with 20% PEG. Blood samples were obtained from a arterial catheter at various time intervals after administration of SB290157; plasma was isolated by centrifugation. Plasma concentrations of SB290157 were quantified by liquid chromatography/mass spectroscopy (MS)/MS (lower limit of quantitation was 10 ng/ml). Noncompartmental methods were used for analysis of plasma concentration vs time data. Induction of arthritis into DBA/1J mice [2] Mice were given 0.2 ml of rat anti-OVA polyclonal antibody (10 mg/ml) by intravenous (i.v.) injection 0.5 h before OVA administration (−0.5 h). OVA (10 µ g) was dissolved in 25 µ l PBS and given by intra-articular (i.a.) injection (0 h). The OVA injection alone was used as the baseline. The net increase in joint thickness attributable to anti-OVA pAb injection was calculated by subtracting the joint thickness of OVA-injected nonimmunized mice from that of the anti-OVA pAb–injected mice. Administration of SB290157, a C3aR antagonist, (10 or 30 mg/kg) was injected i.p. two times, at 0 (right after OVA injection) and 2 h while 5% ethanol in PBS was used as a vehicle control. Joint swelling was measured using a dial thickness gauge before injection, at 0.5 h, and then every hour until 5 h after OVA injection. |
| 药代性质 (ADME/PK) |
The pharmacokinetic profile of SB 290157 was assessed in guinea pigs and mice after i.p. administration. The results of the guinea pig study are summarized in Fig. 5. When administered i.p. at a dose of 30 mg/kg, high and sustained plasma concentrations (>100 ng/ml, 0.25 μM) of SB 290157 were detected out to 8 h (Fig. 5). The Cmax attained was 7000 ng/ml, and the apparent half-life (t1/2) was 0.89 ± 0.26 h. Similar pharmacokinetic data were obtained after i.p. administration of SB 290157 to mice (t1/2 = 1.47 ± 0.10 h; data not shown). [1]
|
| 参考文献 |
|
| 其他信息 |
A small molecule nonpeptide C3aR antagonist, SKF 63649, identified from a high throughput screen inhibited the C3aR binding with low micromolar affinity. After chemical optimization, to afford SB 290157, the affinity for the C3aR was increased by an order of magnitude. SB 290157 was a functional antagonist demonstrating equipotent inhibition of the C3a-induced Ca2+ mobilization response at the native receptor expressed on freshly isolated neutrophils, as well as at the recombinant C3aR stably expressed on RBL-2H3 cells. SB 290157 was a functional antagonist not only of the human C3aR but also of the mouse, rat, and guinea pig C3aRs. The potencies of SB 290157 for inhibition of C3a-induced Ca2+ mobilization of the mouse, guinea pig, and human receptors were similar (IC50 = 7–30 nM). This was somewhat surprising in light of the relatively low level of sequence identity (60–65% overall identity) between the C3aR from these different species. SB 290157 was selective for the C3aR and did not antagonize the C5aR or 5 other chemotactic GPCRs on human neutrophils.
There was good correlation between the antagonist potency of SB 290157 in the human neutrophil Ca2+ mobilization assay and the guinea pig ATP release assay. This result supports the recombinant receptor antagonist data demonstrating similar potency at endogenous C3aRs from two species. However, the antagonist potencies determined in the functional assays were ∼7-fold higher than the affinity estimated in the whole cell binding assay. This is likely due to the inherent differences in the assay protocols, including: differences in times of incubation for the functional assays (seconds) vs the equilibrium conditions (30–60 min) in the binding assay; the temperatures at which the assays were run (room temperature for the binding assay vs 37°C for the functional assay): or possibly the effect of iodination on the affinity of C3a for its receptor. The effect of iodination of C3a on its interaction with the C3aR appears to be minimal, because the affinities determined for C3a with the C3aR in competition binding assays were in good agreement with the published Kd for the C3aR (0.1–1.0 nM). In both binding and functional assays, SB 290157 was consistently 10-fold more potent as a C3aR antagonist than with the initial high throughput screening hit, SKF 63649. The C3aR antagonist compounds had a significant effect on C3a-induced C3aR internalization, inhibiting by almost 50% the number of receptors internalized in response to challenge with 10 nM C3a. At doses of <10 μM, SB 290157 appeared to be a more potent antagonist of C3a-induced receptor internalization than SKF 63649, consistent with the potency obtained with this compound in the binding and functional assays. Marked inhibition of C3a-mediated chemotaxis of HMC-1 cells and of the C3a-induced contractile response to field stimulation in perfused rat caudal arteries was also noted with SB 290157. Concentration response studies were difficult to perform in these assays, but SB 290157 antagonized mouse, rat, and guinea pig C3a receptors with potencies equivalent to the potency vs the human C3aR. These data, combined with the determination that after i.p. administration to mice and guinea pigs plasma levels of SB 290157 were high and sustained, indicated that it was a suitable compound for study in animal models to help define the physiological and pathophysiological role of C3a and the C3aR. We studied the C3aR antagonist, SB 290157, in two animal models of inflammation. In the first, SB 290157 inhibited neutrophil recruitment and accumulation in a guinea pig LPS-induced airway neutrophilia model. The inhibitory activity appeared to be specific for neutrophils as the number of neutrophils recovered in the challenged lungs was decreased, but there was no significant inhibition of the total number of cells recovered. This is somewhat surprising because C3a is not chemotactic for neutrophils, although they express the C3aR, demonstrate specific binding, and respond to C3a with a transient calcium response. The effect of SB 290157 may be a secondary rather than a direct effect on neutrophil recruitment. SB 290157 was also tested in a disease-modifying rat model of AIA. Antiinflammatory activity was observed in Lewis rats that received SB 290157, 30 mg/kg i.p. b.i.d. There was a significant reduction (41%) in paw swelling as compared with the control untreated animals. This is significant activity for the C3aR antagonist in an aggressive arthritis model and potentially implicates C3a in the pathogenesis of this disease. Our data indicate that SB 290157 is a high affinity, selective, and competitive C3aR antagonist. It is active in two in vivo models of inflammation; therefore, it shows promise as a tool compound for further studies to elucidate physiological and pathophysiological role(s) of C3aR activation. [1] In conclusion, the present data indicate that SB 290157 can inhibit the induction of arthritis by lowering the level of joint swelling, neutrophil migration, and IL-1 β production. The current findings suggested that C3a may be involved in the aggravation of arthritis by using SB 290157. Therefore, SB 290157 may be effective in the inhibition of arthritis. [2] |
| 分子式 |
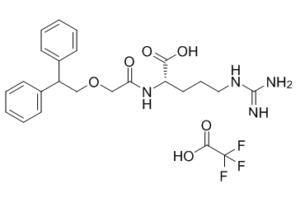
C24H29F3N4O6
|
|---|---|
| 分子量 |
526.505476713181
|
| 精确质量 |
526.203
|
| 元素分析 |
C, 54.75; H, 5.55; F, 10.83; N, 10.64; O, 18.23
|
| CAS号 |
1140525-25-2
|
| 相关CAS号 |
1140525-25-2 (TFA);259218-28-5;
|
| PubChem CID |
16760645
|
| 外观&性状 |
White to yellow solid powder
|
| tPSA |
177
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
619
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC=C(C=C1)C(COCC(=O)N[C@@H](CCCN=C(N)N)C(=O)O)C2=CC=CC=C2.C(=O)(C(F)(F)F)O
|
| InChi Key |
ZJRMPPVJAQWGEG-FYZYNONXSA-N
|
| InChi Code |
InChI=1S/C22H28N4O4.C2HF3O2/c23-22(24)25-13-7-12-19(21(28)29)26-20(27)15-30-14-18(16-8-3-1-4-9-16)17-10-5-2-6-11-17;3-2(4,5)1(6)7/h1-6,8-11,18-19H,7,12-15H2,(H,26,27)(H,28,29)(H4,23,24,25);(H,6,7)/t19-;/m0./s1
|
| 化学名 |
(2S)-5-(diaminomethylideneamino)-2-[[2-(2,2-diphenylethoxy)acetyl]amino]pentanoic acid;2,2,2-trifluoroacetic acid
|
| 别名 |
1140525-25-2; SB290157 trifluoroacetate; SB 290157 trifluoroacetate salt; SB290157 (trifluoroacetate); (2S)-5-(diaminomethylideneamino)-2-[[2-(2,2-diphenylethoxy)acetyl]amino]pentanoic acid;2,2,2-trifluoroacetic acid; SB 290157 trifluoroacetate; N2-[2-(2,2-Diphenylethoxy)acetyl]-L-arginine 2,2,2-Trifluoroacetate;; SB290157 trifluoroacetate salt;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~189.93 mM)
Ethanol : ~100 mg/mL (~189.93 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 5 mg/mL (9.50 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,将 100 μL 50.0 mg/mL 澄清乙醇储备液加入到 400 μL PEG300 中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 5 mg/mL (9.50 mM) (饱和度未知) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL 澄清乙醇储备液加入到 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 5 mg/mL (9.50 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (3.95 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清的DMSO储备液加入400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (3.95 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 2.08 mg/mL (3.95 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8993 mL | 9.4965 mL | 18.9930 mL | |
| 5 mM | 0.3799 mL | 1.8993 mL | 3.7986 mL | |
| 10 mM | 0.1899 mL | 0.9496 mL | 1.8993 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。