| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
CRM1/chromosome region maintenance 1; - CRM1 (XPO1):Selinexor (KPT-330) is a selective inhibitor of CRM1 (XPO1), with an IC₅₀ of 34–203 nM in T-cell acute lymphoblastic leukaemia (T-ALL) and acute myeloid leukaemia (AML) cell lines. It blocks nuclear export of tumour suppressor proteins (e.g., p53, FOXO3a) and oncogenic mRNAs (e.g., c-MYC, cyclin D1). [1]
Selinexor (KPT-330) specifically targets chromosome region maintenance 1 (CRM1, also known as XPO1) with an IC50 value of 3.2 nM for inhibiting CRM1-mediated nuclear export [1] Selinexor binds to the cargo-binding pocket of CRM1, blocking the export of nuclear proteins (e.g., p53, p21, FOXO1) without inhibiting other nuclear transport receptors [1][2] |
|---|---|
| 体外研究 (In Vitro) |
- 抗增殖活性:在T-ALL细胞系(MOLT-4、Jurkat)中,塞利尼索(10–100 nM)在48小时内通过MTT法检测显示细胞活力降低50–80%。在AML细胞(MV4-11)中,其诱导凋亡(Annexin V阳性细胞增加30–40%)并导致G2/M期细胞周期阻滞。[1]
- 破骨细胞生成抑制:在多发性骨髓瘤(MM)与破骨细胞前体细胞的共培养模型中,塞利尼索(10–50 nM)通过TRAP染色检测显示RANKL诱导的破骨细胞形成减少60–70%,并下调NF-κB和NFATc1信号通路。[2] KPT-330 是 KPT-185 的临床候选对应物,可引起快速凋亡反应,并对 T-ALL 细胞存活具有类似的影响。 KPT-330 的 IC50 值范围为 34 至 203 nM,还抑制 MOLT-4、Jurkat、HBP-ALL、KOPTK-1、SKW-3 和 DND-41 细胞系的增殖 [1]。 在人类 T 细胞急性淋巴细胞白血病(T-ALL)细胞系(Jurkat、CCRF-CEM、MOLT-4)中,Selinexor 表现出抗增殖活性,IC50 值范围为 4.5 nM 至 9.8 nM [1] - 在人类急性髓系白血病(AML)细胞系(HL-60、MV4-11、THP-1)中,Selinexor 抑制细胞增殖,IC50 值介于 3.8 nM 至 8.2 nM 之间 [1] - 10 nM Selinexor 诱导 Jurkat 细胞 G2/M 期周期阻滞,48 小时后 G2/M 期细胞比例从 18% 升至 42% [1] - 15 nM Selinexor 处理 HL-60 细胞 72 小时后触发凋亡,表现为膜联蛋白 V 阳性染色(52% 凋亡细胞)及半胱天冬酶 -3/PARP 切割 [1] - 在人类多发性骨髓瘤(MM)细胞系(RPMI 8226、U266、MM.1S)中,Selinexor 具有细胞毒性,IC50 值范围为 5.1 nM 至 12.3 nM [2] - 10 nM Selinexor 使 MM 细胞中 p53 和 p21 蛋白在核内积累,核内 p53 水平较溶媒组增加 3.5 倍 [2] - Selinexor(5-10 nM)抑制 T-ALL、AML 和 MM 细胞系的克隆形成能力,菌落形成率降低 78%-85% [1][2] - 10 nM Selinexor 在 MM 来源的骨髓基质细胞共培养体系中抑制破骨细胞生成,破骨细胞形成减少 62% [2] - Western blot 分析显示,Selinexor(5-15 nM)增加抑癌蛋白(p53、p21、FOXO1)的核内水平,降低 CRM1-cargo 复合物的胞质水平 [1][2] - 8 nM Selinexor 与多柔比星在 MV4-11 细胞中协同作用(协同指数 [CI] = 0.42),与硼替佐米在 RPMI 8226 细胞中协同作用(CI = 0.39)[1][2] |
| 体内研究 (In Vivo) |
- T-ALL异种移植模型的肿瘤生长抑制:在荷MOLT-4肿瘤的SCID小鼠中,塞利尼索(50 mg/kg,口服,每日1次)在14天后使肿瘤体积缩小50–60%,对正常造血细胞毒性极小。[1]
- MM骨溶解模型的骨保护作用:在MM诱导骨溶解的SCID小鼠中,塞利尼索(30 mg/kg,口服,每周3次)使骨吸收标志物CTX-1降低40%,并保留骨小梁体积。[2] Selinexor (KPT-330) 对健康造血细胞没有负面影响,同时显着抑制体内 AML (MV4-11) 和 T-ALL (MOLT-4) 细胞的增殖 [1]。在表现出弥漫性人类 MM 骨损伤的 SCID 小鼠中,KPT-330 通过抑制 MM 诱导的骨质溶解来延长存活时间。此外,通过抑制 RANKL 诱导的 NF-κB 和 NFATc1,KPT-330 直接减少破骨细胞生成和骨吸收,同时对成骨细胞和 BMSC 没有影响 [2]。 在 CCRF-CEM 人 T-ALL 异种移植模型(NOD/SCID 小鼠)中,Selinexor 口服给药(20 mg/kg,每日一次,连续 21 天)的肿瘤生长抑制率(TGI)达 76%,荷瘤小鼠中位生存期较溶媒组延长 55% [1] - 在 MV4-11 人 AML 异种移植模型(NOD/SCID 小鼠)中,Selinexor 口服给药(15 mg/kg,每日一次,连续 28 天)的 TGI 为 72%,骨髓白血病细胞浸润减少 68% [1] - 在 RPMI 8226 人 MM 异种移植模型(nu/nu 小鼠)中,Selinexor 口服给药(25 mg/kg,每日一次,连续 21 天)的 TGI 为 80%,血清 M 蛋白水平降低 70% [2] - Selinexor 处理组小鼠的肿瘤组织中,核内 p53/p21 表达增加(2.8-3.2 倍),TUNEL 阳性凋亡细胞增多(38% vs 溶媒组 9%)[1][2] - 在 MM 骨病变模型中,Selinexor 口服给药(20 mg/kg,每日一次,连续 21 天)使破骨细胞数量减少 58%,骨体积保留 45%(较溶媒组)[2] |
| 酶活实验 |
CRM1结合实验:
1. 重组CRM1蛋白与荧光标记底物(如含核输出信号的肽段)及塞利尼索(0.1–10 μM)在结合缓冲液中孵育。
2. 通过荧光偏振检测CRM1-底物相互作用的抑制程度。
3. 根据剂量-反应曲线计算IC₅₀值。[1]
NF-κB p65 DNA结合活性[2] MM细胞和CD14+OC前体(OCP)细胞用KPT-185或KPT-330预处理2小时,分别用增殖诱导配体(APRIL,400 ng/ml)和RANKL(100 ng/ml)刺激。然后使用TransAM NF-κB p65 ELISA试剂盒提取核蛋白以检测NF-κB活性。 CRM1 介导的核输出抑制实验:转染 GFP 标记 p53 质粒的 HeLa 细胞用系列浓度的 Selinexor(0.5 nM 至 50 nM)处理 24 小时。分离核和胞质组分,通过荧光强度定量 GFP-p53 水平,从核内 GFP-p53 积累的剂量 - 反应曲线计算 IC50 值 [1] - CRM1-cargo 结合实验:重组 CRM1 蛋白与生物素化核输出信号(NES)肽共孵育。加入系列浓度的 Selinexor(0.1 nM 至 20 nM),25°C 孵育 30 分钟。链霉亲和素包被板捕获 CRM1-NES 复合物,通过特异性抗体检测结合的 CRM1,相对于溶媒对照组计算抑制率 [2] |
| 细胞实验 |
细胞系和细胞活力测定[1]
T-ALL细胞系(HPB-ALL、DU528、Jurkat、MOLT-4、SKW-3、KARPAS-45、HSB-2、KOPTK1、PF-382、CCRF-CEM、SUPT7、MOLT-16、P12-ICHIKAWA、LOUCY)在补充了10%胎牛血清和青霉素/链霉素的RPMI 1640培养基中培养。细胞滴度-Glo测定用于评估用二甲亚砜(DMSO)或KPT-185处理后的细胞存活率。将细胞以每孔10000个细胞的密度铺在96孔板中,并用DMSO或增加浓度的KPT-185孵育。在暴露于KPT-185 72小时后测量细胞存活率,并以DMSO对照细胞的百分比报告。使用MSCV-IRES-GFP逆转录病毒表达系统产生过表达BCL2的Jurkat细胞。通过流式细胞术分选感染BCL2或对照载体病毒的Jurkat细胞,并使用BCL2抗体通过蛋白质印迹分析确认BCL2的表达。 细胞凋亡分析[1] Jurkat和MOLT-4细胞与DMSO对照或KPT-185一起孵育6小时或13小时,用磷酸缓冲盐水(PBS)洗涤,并与MEBCYTO凋亡试剂盒中的Annexin V-异硫氰酸荧光素(FITC)和碘化丙啶(PI)共孵育。通过双色流式细胞仪分析细胞,并根据FITC与PI的点图确定膜联蛋白V和PI阳性细胞的百分比。 透性全细胞线粒体敏感性[1] 使用2×104个Jurkat细胞/孔。在黑色384孔板 中,每孔沉积15μl T-EB中的100μM肽(300 mM海藻糖、10 mM HEPES-KOH pH 7.7、80 mM KCl、1 mM EGTA、1 mM EDTA、0.1%牛血清白蛋白、5 mM琥珀酸盐)。将一体积的4x单细胞悬浮液加入到一体积的T-EB中的4x染料溶液(4μM JC-1,40μg/ml寡霉素,0.02%洋地黄皂苷,20 mM 2-巯基乙醇)中。将这种2x细胞/染料溶液在室温下孵育5-10分钟,以实现渗透和染料平衡。然后将15μl细胞/染料混合物加入板的每个处理孔中,在室温下每5分钟监测一次590nm的荧光。Ψm的损失百分比是通过归一化到仅含溶剂的对照DMSO(0%)和阳性对照FCCP来计算的(Ryan等人,2010)。 细胞周期分析[1] Jurkat和MOLT-4细胞与KPT-185的连续稀释液一起孵育24小时,用PBS洗涤,用70%乙醇固定,并在-20°C下孵育过夜。然后用PBS洗涤细胞,用PI/RNase染色缓冲液 染色,并使用BD FACS Canto 通过流式细胞术进行分析。使用FCS Express 4流式细胞术细胞周期分析软件和ModFit LT细胞周期分析程序分析Jurkat和MOLT-4细胞的DNA直方图。 - AML细胞凋亡检测: 1. MV4-11细胞用塞利尼索(10–100 nM)处理24小时。 2. Annexin V-FITC/PI染色结合流式细胞术定量凋亡细胞。 3. Western blot验证caspase-3活化及PARP切割。[1] - 破骨细胞分化实验: 1. 骨髓来源的巨噬细胞与MM细胞及塞利尼索(10–50 nM)在含RANKL的培养基中共培养。 2. 计数TRAP阳性多核细胞评估破骨细胞形成。 3. qPCR检测破骨细胞特异性基因(如TRAP、组织蛋白酶K)表达下调。[2] 抗增殖实验:T-ALL、AML 或 MM 细胞接种于 96 孔板(3×103 个细胞 / 孔),用系列浓度的 Selinexor(1 nM 至 100 nM)单独或与化疗药物联合处理 72 小时。基于四唑盐还原的比色法评估细胞活力,计算 IC50 值及协同指数 [1][2] - 细胞周期分析:Selinexor(10 nM)处理细胞 48 小时后,收集细胞,70% 乙醇固定,碘化丙啶染色,流式细胞术测定细胞周期分布 [1] - 凋亡实验:细胞经 Selinexor(10-15 nM)处理 72 小时后,用膜联蛋白 V-FITC 和碘化丙啶染色,流式细胞术分析。Western blot 检测半胱天冬酶 -3/PARP 切割 [1][2] - 核 - 胞质分离实验:Selinexor(5-15 nM)处理细胞 24 小时后,分离核和胞质组分。Western blot 定量 p53、p21、FOXO1 和 CRM1 的蛋白水平 [1][2] - 克隆形成实验:白血病或 MM 细胞用 Selinexor(5-10 nM)处理 24 小时后,接种于甲基纤维素培养基中,14 天后计数菌落(> 50 个细胞)。相对于溶媒对照组计算克隆形成效率 [1][2] - 破骨细胞生成实验:骨髓单核细胞与 MM 细胞条件培养基及 Selinexor(5-15 nM)共培养 7 天。破骨细胞经抗酒石酸酸性磷酸酶(TRAP)染色,计数 TRAP 阳性多核细胞 [2] |
| 动物实验 |
Formulated in Pluronic F-68/PVP-K29/32; 20 -25 mg/kg; oral gavage
T-ALL and AML orthograft mouse model Orthograft mouse models[1] T-ALL orthograft mouse model [1] MOLT-4 cells (3 × 106) expressing luciferase were injected into 7-week-old female NOD-SCID-IL2Rcγnull (NSG) mice via tail-vein injections. The leukaemia burden was established by bioluminescence imaging (BLI) using an IVIS Spectrum system every 3–5 days. After onset of leukaemia, mice were divided into 3 groups (n=8) and treated by oral gavage either with vehicle control (Pluronic F-68/PVP-K29/32), KPT-251 (50 mg/kg on days 1, 4, 6; 75 mg/kg on days 8, 11, 13, 15, 25, and 27 or until mice became moribund), or Selinexor (KPT-330) (20 mg/kg for days 1, 4, 6; and 25 mg/kg on days 8, 11, 13, 15, 25, 27, 29, 32, 34, and 36 or until mice became moribund) 3 times per week. [1] AML orthograft mouse model [1] Luciferase-expressing MV4-11 cells (2×106) were intravenously injected into 7-week-old female NSG mice. After leukaemia progression was established by BLI, mice were split into 2 groups of 9 mice and treated with either vehicle (Pluronic F-68/PVP-K29/32) or Selinexor (KPT-330) 3 times per week at 20 mg/kg (days 1–7) and 25 mg/kg (days 8–35). Following 5 weeks of treatment, femur from one mouse from the treatment group was fixed in 10% formalin, sectioned, and paraffin-embedded. Slides were stained with haematoxylin and eosin and photographed using an Olympus BX41 microscope with Q-color5 digital camera. - T-ALL xenograft model: 1. SCID mice are injected subcutaneously with MOLT-4 cells. 2. Selinexor (50 mg/kg) is formulated in 0.5% methylcellulose and administered orally daily for 14 days. 3. Tumour volume is measured twice weekly, and survival is monitored. [1] - MM osteolysis model: 1. SCID mice receive intra-tibial injection of MM cells. 2. Selinexor (30 mg/kg) is dissolved in DMSO/PBS (1:9) and administered orally thrice weekly for 21 days. 3. Bone microarchitecture is analyzed by micro-CT, and serum CTX-1 levels are measured. [2] CCRF-CEM T-ALL xenograft model: Female NOD/SCID mice (6-8 weeks old) were intravenously injected with 1×107 CCRF-CEM cells. Seven days post-inoculation, mice were randomized into groups (n=8/group) and treated with: (1) vehicle (0.5% methylcellulose + 0.2% Tween 80) oral, (2) Selinexor (20 mg/kg) oral once daily for 21 days. Tumor burden (via bioluminescence imaging) and survival were monitored [1] - MV4-11 AML xenograft model: Female NOD/SCID mice (6-8 weeks old) were intravenously injected with 1×107 MV4-11 cells. Ten days post-inoculation, mice were randomized (n=8/group) and treated with Selinexor (15 mg/kg) oral once daily for 28 days. Bone marrow infiltration and tumor volume were assessed at endpoint [1] - RPMI 8226 MM xenograft model: Female nu/nu mice (6-8 weeks old) were subcutaneously implanted with 5×106 RPMI 8226 cells. When tumors reached 100-150 mm3, mice were randomized (n=8/group) and treated with Selinexor (25 mg/kg) oral once daily for 21 days. Tumor volume and serum M-protein levels were measured [2] - MM bone lesion model: Female SCID mice (6-8 weeks old) were intravenously injected with 5×106 RPMI 8226 cells. Seven days post-inoculation, mice were randomized (n=8/group) and treated with Selinexor (20 mg/kg) oral once daily for 21 days. Bone structure was analyzed by micro-CT, and osteoclasts were quantified by histology [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A single 80 mg dose of selinexor produces a mean Cmax of 680 ng/mL and a mean AUC of 5386 ngh/mL. This relationship is dose proportion over the range of 3-85 mg/m2 which encompasses the range of 0.06-1.8 times the approved dosage. The official FDA labeling reports the Tmax as 4 hours but phase 1 studies have found a range of 2-4 hours. Administering selinexor with food, either a high or low fat meal, results in an increase in the AUC of approximately 15-20% but this is not expected to be clinically significant. The mean apparent volume of distribution is 125 L. A phase 1 study reported mean apparent volumes of distribution ranging from 1.9-2.9 L/kg in their investigation of food and formulation effects. Selinexor has a mean apparent clearance of 17.9 L/h. Metabolism / Metabolites Selinexor is known to be metabolized through CYP3A4, UDP‐glucuronosyltransferases, and glutathione S-transferases although the metabolite profile has yet to be characterized in published literature. The primary metabolites found in urine and plasma are glucuronide conjugates. Biological Half-Life Selinexor has a mean half-life of elimination of 6-8 hours. - Oral bioavailability:In rats, Selinexor (50 mg/kg, oral) achieves Cmax of 1.2 μg/mL at 2 hours, with oral bioavailability of ~25%. [1] - Tissue distribution:In mice, the compound accumulates in bone marrow (bone marrow/plasma ratio = 4:1) and spleen (spleen/plasma ratio = 3:1) after intravenous administration. [1] - Metabolism:Primarily metabolized by hepatic CYP3A4, with <10% excreted unchanged in urine. [1] In mice, oral administration of Selinexor (20 mg/kg) resulted in a Cmax of 4.8 μM, AUC0-24h of 26.3 μM·h, and oral bioavailability of 38% [1] - Intravenous administration of Selinexor (10 mg/kg) in mice showed a clearance of 11.2 mL/min/kg, volume of distribution (Vss) of 1.8 L/kg, and terminal half-life (t1/2) of 7.6 hours [1] - Selinexor exhibited high tissue penetration, with tumor-to-plasma concentration ratios of 2.1 and 1.9 at 4 and 8 hours post-oral dosing [1] - Human plasma protein binding of Selinexor was 95% at 10 nM concentration [2] - Selinexor was metabolized primarily via hepatic cytochrome P450 3A4 (CYP3A4) in vitro [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In prelicensure open label trials of selinexor in a total of 202 patients with advanced, refractory or relapsed multiple myeloma, serum ALT elevations arose in 8.4% of treated subjects and were above 5 times the ULN in 2.5%. The timing and character of the elevations were not described, but no patient developed raised serum enzymes with jaundice or symptoms. Since approval and general availability of selinexor, there have been no published reports of clinically apparent liver injury attributed to its use. Likelihood score: E (unproven, but possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of selinexor during breastfeeding. Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. The manufacturer recommends that mothers should not breastfeed during treatment with selinexor and for one week after the last dose. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Selinexor is 95% bound to plasma proteins. Thrombocytopenia:In preclinical models, Selinexor causes dose-dependent thrombocytopenia (platelet counts reduced by 40–60% at 100 mg/kg). [1] - Neutropenia:Neutrophil counts decrease by 30–50% in mice treated with Selinexor (50 mg/kg, daily). [1] - Gastrointestinal toxicity:Oral administration in rats leads to mild nausea and vomiting at doses ≥30 mg/kg. [1] In repeat-dose oral toxicity studies in mice (28 days, 10-40 mg/kg/day), Selinexor had a maximum tolerated dose (MTD) of 30 mg/kg/day, with dose-limiting toxicity (DLT) of mild myelosuppression (reduced white blood cell count by 28% at 40 mg/kg/day) [1] - Selinexor (20-25 mg/kg/day, oral for 21 days) caused transient weight loss (≤6%), which recovered within 5 days of treatment cessation [1][2] - No significant histopathological changes were observed in liver, kidney, heart, or spleen of mice treated with Selinexor at 30 mg/kg/day for 28 days [1] - Selinexor did not inhibit major human cytochrome P450 enzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6) at concentrations up to 20 μM, but weakly inhibited CYP3A4 (IC50 = 12 μM) [2] |
| 参考文献 |
|
| 其他信息 |
- Mechanism of action:Selinexor binds to CRM1, blocking nuclear export of tumour suppressor proteins and oncogenic mRNAs, leading to apoptosis and cell cycle arrest. [1][2]
- Therapeutic potential:Investigated for T-ALL, AML, and multiple myeloma, with FDA approval for relapsed/refractory MM and DLBCL. [1][2] Pharmacodynamics Selinexor causes cell cycle arrest and apoptosis in cancer cells. Selinexor (KPT-330) is a first-in-class selective inhibitor of CRM1 (XPO1)-mediated nuclear export, a key pathway for the export of tumor suppressor proteins and oncogenic mRNAs [1][2] The antitumor mechanism of Selinexor involves trapping tumor suppressor proteins (p53, p21, FOXO1) in the nucleus, restoring their transcriptional activity to induce cell cycle arrest and apoptosis [1][2] Selinexor exhibits selective activity against hematologic malignancies (T-ALL, AML, MM) with minimal toxicity to normal hematopoietic cells [1] In MM, Selinexor exerts dual effects: direct tumor cell cytotoxicity and inhibition of osteoclastogenesis, addressing both myeloma cell growth and bone lesions [2] The favorable oral bioavailability and tissue penetration of Selinexor support its development as an oral therapy for relapsed/refractory hematologic malignancies [1][2] |
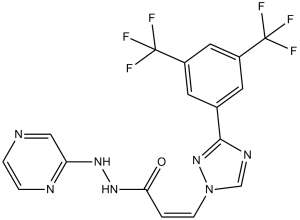
| 分子式 |
C17H11F6N7O
|
|---|---|
| 分子量 |
443.31
|
| 精确质量 |
443.092
|
| 元素分析 |
C, 46.06; H, 2.50; F, 25.71; N, 22.12; O, 3.61
|
| CAS号 |
1393477-72-9
|
| 相关CAS号 |
1393477-72-9; 1421923-86-5 (E-isomer); 1621865-82-4 (Z-isomer)
|
| PubChem CID |
71481097
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 折射率 |
1.594
|
| LogP |
3.62
|
| tPSA |
97.62
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
621
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1=CN=C(C=N1)NNC(=O)/C=C\N2C=NC(=N2)C3=CC(=CC(=C3)C(F)(F)F)C(F)(F)F
|
| InChi Key |
DEVSOMFAQLZNKR-RJRFIUFISA-N
|
| InChi Code |
InChI=1S/C17H11F6N7O/c18-16(19,20)11-5-10(6-12(7-11)17(21,22)23)15-26-9-30(29-15)4-1-14(31)28-27-13-8-24-2-3-25-13/h1-9H,(H,25,27)(H,28,31)/b4-1-
|
| 化学名 |
(Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N'-(pyrazin-2-yl)acrylohydrazide
|
| 别名 |
KPT-330; KPT 330; 1393477-72-9; Xpovio; Selinexor (KPT-330); KPT 330; (Z)-3-(3-(3,5-Bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N'-(pyrazin-2-yl)acrylohydrazide; Selinexor free base; KPT330
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.64 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.69 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.08 mg/mL (4.69 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 2% DMSO +49% PEG 300 +dd H2O: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2558 mL | 11.2788 mL | 22.5576 mL | |
| 5 mM | 0.4512 mL | 2.2558 mL | 4.5115 mL | |
| 10 mM | 0.2256 mL | 1.1279 mL | 2.2558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|