| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
PARP1/2
|
|---|---|
| 体外研究 (In Vitro) |
Senaparib是一种新型的选择性聚ADP核糖聚合酶-1/2抑制剂,在临床前研究中具有很强的抗肿瘤活性。 Senaparib (IMP4297) 正在研究作为胰腺癌、乳腺癌和晚期肝癌的治疗方法 [2]。Senaparib是一种口服生物可利用的核酶聚ADP核糖聚合酶(PARP)1和2抑制剂,具有潜在的抗肿瘤活性。给药后,senaparib选择性结合PARP 1和2,并通过碱基切除修复途径阻止PARP介导的单链DNA断裂的DNA修复。这增强了DNA链断裂的积累,促进了基因组的不稳定性,最终导致细胞凋亡。PARP催化核蛋白的翻译后ADP核糖基化,核蛋白发出信号并招募其他蛋白质来修复受损的DNA,并被单链DNA断裂激活。
|
| 体内研究 (In Vivo) |
Senaparib(前身为IMP4297)是一种新型的选择性口服PARP1和PARP2抑制剂,在临床前研究中显示出很强的抗肿瘤活性,体内活性比奥拉帕尼(目前批准的PARP中开发最完善的一种)高20倍。 Senaparib的第一阶段人体研究是在澳大利亚晚期实体瘤患者中进行的(ClinicalTrials.gov标识符NCT03507543)。研究了单剂量和多剂量番泻叶的安全性、耐受性和药代动力学(PK)特征,并记录了初步的抗肿瘤反应。[1]
39名患者以2至150mg的10个剂量水平入组。在任何队列中均未观察到剂量限制性毒性。大多数治疗中出现的不良事件为1-2级(91%)。7名患者(17.9%)报告了血液治疗中出现的不良事件。8名患者(20.5%)发生了与治疗相关的不良事件,最常见的是恶心(7.7%)。研究治疗结束后报告了两例死亡,其中一例被认为是与Senaparib相关的骨髓衰竭引起的并发症。药代动力学分析表明,senaparib的累积指数为1.06-1.67,吸收饱和度为每天80-150mg。在22名可评估疾病的患者中,总有效率为13.6%,疾病控制率为81.8%。BRCA突变阳性亚组的总体反应率为33.3%,非突变亚组为6.3%。 结论:Senaparib在澳大利亚晚期实体瘤患者中具有良好的耐受性,具有令人鼓舞的抗肿瘤活性信号。senaparib的推荐2期剂量确定为每日100mg。[1] 疗效: 在根据RECIST 1.1标准可评估肿瘤反应的22名患者中,有6名患者被确认为BRCA1或BRCA2突变携带者(见表S12)。在这22名患者中,有3名患者(均为癌症患者)出现PR(20 mg、100 mg和120 mg剂量组各1例),ORR为13.6%(95%CI,2.9%–34.9%)。其中两名应答者患有BRCA突变阳性肿瘤(20mg和100mg剂量组各一例),BRCA突变阴性亚组的ORR为33.3%(六名患者中有两名;95%CI,4.3%-77.7%)。非突变亚组的ORR为6.3%(16名患者中的一名)。另外15名患者(68.2%)总体上患有SD。DCR总体上为81.8%(95%CI,59.7%-94.8%),与BRCA突变阳性亚组相似(83.3%;95%CI,35.9%-99.6%)。在100mg组中,ORR为20%(95%置信区间,0.5%-71.6%),DCR为40%(95%置信范围,5.3%-85.3%)。图3显示了所有可评估患者的目标病变大小最佳变化的瀑布图。在数据截止日期,所有三名应答者仍然活着,没有疾病进展,BRCA野生型患者的应答持续时间为1.4个月,两名BRCA突变阳性患者的应答时间为2.8个月和22.1个月。在疗效人群中,中位无进展生存期为5.7个月(95%置信区间,2.7%-7.4%),在BRCA突变阳性亚组中为7.4个月(95%CI,1.77%未达到)(见表S13和图S2)。10名癌症前列腺患者中有1名(10%)出现PSA反应;他患有BRCA野生型,属于40mg剂量组[1]。 |
| 动物实验 |
Study design [1]
Details of the dose-escalation protocol can be found in the Supporting Methods. Patients were initially administered one dose of oral Senaparib; after a 7-day washout period, senaparib was administered once daily in 3-week cycles (from day 1 [D1] to D21). If no dose-limiting toxicity (DLT) emerged, the dose was increased from 6 to 40 mg once daily in dose cohorts in a stepwise manner. For subsequent dose levels, the study followed a conventional 3 + 3 design24 to determine the maximum tolerated dose (MTD; the maximum dose at which one in six patients from a single cohort experienced a DLT during the first treatment cycle [C1]) or the recommended phase 2 dose (RP2D; based on the toxicity end point—the MTD or one dose level below; Figure S1 and Table S2). Treatment with Senaparib continued for up to 1 year until disease progression or unacceptable toxicity or until the investigator determined there was no benefit to the patient. This study was conducted in accordance with the protocol, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use good clinical practice guidelines, applicable regulations and guidelines governing clinical study conduct, and the ethical principles originating in the Declaration of Helsinki. All patients provided written informed consent to participate before their inclusion in the study. End points [1] Primary end points were the incidence and nature of DLTs, and the incidence, nature, relatedness, and severity of treatment-emergent adverse events (TEAEs). The secondary end point was the PK parameters of Senaparib. Exploratory efficacy end points were the overall response rate (ORR), the disease control rate (DCR; complete responses [CRs] + partial responses [PRs] + stable disease [SD] lasting ≥6 weeks), the duration of response, progression-free survival (PFS), and, where applicable, serum prostate-specific antigen (PSA) and cancer antigen 125 (CA-125) concentrations. A full list of all efficacy end points and their definitions can be found in Table S3. Study assessments [1] TEAEs and serious adverse events (SAEs) were recorded throughout the study, and patients were followed for safety for 30 days after the last dose of Senaparib or at treatment discontinuation, whichever occurred later. All TEAEs were graded for severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03),25 and their relatedness was investigator assessed according to protocol-defined criteria Tables (see S4 and S5). Dose modifications to manage any toxicities were allowed after C1 (see Table S6). The window for DLT assessment was C1D1 to C1D21. DLTs were defined as the occurrence of any of the following during the assessment window: any grade ≥3 nonhematologic toxicity, grade 4 neutropenia lasting >7 days, febrile neutropenia (absolute neutrophil count [ANC] <1000 cells/mm3 and fever ≥38.5°C) or documented grade ≥3 infection with an absolute neutrophil count ≤1000 cells/mm3, grade 4 thrombocytopenia lasting >48 hours or requiring intervention or associated with increased bleeding, or dose interruption for >14 days because of toxicity. Any patient experiencing a DLT was treated according to standard clinical practice and discontinued from the study treatment. Blood sampling for measurement of PK and PSA/CA-125 concentrations and assessments for antitumor efficacy are described in the Supporting Methods. Antitumor efficacy was assessed in patients with a measurable lesion using Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 |
| 药代性质 (ADME/PK) |
Pharmacokinetics [1]
The senaparib single-dose PK data are presented in Figure 2A and Table S10. The median time to reach Cmax of senaparib was 1.00–2.08 hours across dose levels. Senaparib exposure parameters (Cmax and AUC) demonstrated an increasing trend with increasing doses in the dose range from 2 to 80 mg but were comparable in the range from 80 to 150 mg. The relationships between dose and senaparib exposure supported a plateau commencing at 80 mg daily. Data for the senaparib multiple-dose PK parameters are presented in Figure 2B and Table S11. The PKs of senaparib after multiple-dose administration followed the same pattern as the single-dose administration (Figure 2B). The median time to reach Cmax of senaparib was 1.97–2.13 hours after single-dose administration of 2–150 mg during this multiple-dose stage (D1). The mean elimination half-life ranged from 5.86 to 13.30 hours for D1 and from 5.68 to 8.39 hours for D15. There was no apparent accumulation of senaparib in the body after multiple dosing (accumulation index, 1.06–1.67). |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and tolerability [1]
Overall, 38 patients (97.4%) experienced at least one TEAE (267 events in total; Table 2). The incidence and severity of TEAEs did not appear to be dose-dependent. The most common TEAEs of any grade were fatigue, headache (n = 10; 25.6% for each), and nausea (n = 9; 23.1%; Table 3). The majority of TEAEs were grade 1 or 2 (n = 25; 64.1%; Table 2). TEAEs resulted in dose discontinuation or interruption in six patients (15.4%) and eight patients (20.5%), respectively. Two deaths were reported, and both occurred after the end of study treatment. One death was attributed to progression of metastatic breast cancer and was considered unrelated to senaparib: the patient died 27 days after the withdrawal of treatment. The other death occurred to a patient with non-BRCA–mutated ovarian cancer, and it was attributed to a grade 5 event of bone marrow failure related to senaparib, for which the bone marrow biopsy did not indicate myelodysplastic syndrome (MDS); this patient also had grade 3 anemia, grade 4 neutropenia and grade 4 thrombocytopenia. The patient had a response of SD to the treatment at dose level of 80 mg daily, 10.9 months free of disease progression, and died 96 days after the withdrawal of treatment. Eight patients (20.5%) reported treatment-related AEs; the most common were nausea (n = 3; 7.7%), fatigue, and thrombocytopenia (n = 2; 5.1% for each; see Table S8). In total, 15 patients (38.5%) experienced 28 SAEs (see Table S9), of which 22 events (78.6%) in 14 patients were grade 2 or 3. The most frequent SAEs were hematuria (two events in two patients [5.1%], both grade 3) and pulmonary embolism (two events in two patients [5.1%], one each at grades 2 and 3). Almost all reported SAEs were considered either not related or unlikely to be related to senaparib; the exception was the SAE of grade 5 bone marrow failure already mentioned. Hematologic TEAEs occurred in seven patients (17.9%). Anemia was reported in four patients (10.3%; three at grade 2 and one at grade 3), thrombocytopenia in three patients (7.7%; two at grade 1 and one at grade 3), and neutropenia in one patient (2.6%; grade 4). The final hematologic TEAE was the grade 5 bone marrow failure, which was considered to be probably related to the study drug. This patient was diagnosed with a grade 4 SAE of decreased platelet count on study day 239, leading to study drug discontinuation, and was further diagnosed with bone marrow failure on study day 263, leading to death on day 353. There were no cases of secondary hematologic malignancies among the patients in this study. No DLTs were observed during the protocol-defined DLT period at any dose level. Therefore, the MTD was not reached. Considering the absorption of senaparib tended to be saturated during the 80–150 mg dose range, and the preliminary efficacy was a 20% ORR at 100 mg, the RP2D of senaparib was determined to be 100 mg daily. |
| 参考文献 |
|
| 其他信息 |
Senaparib is an orally bioavailable inhibitor of the nuclear enzymes poly (ADP-ribose) polymerase (PARP) 1 and 2, with potential antineoplastic activity. Upon administration, senaparib selectively binds to PARP 1 and 2 and prevents PARP-mediated DNA repair of single-strand DNA breaks via the base-excision repair pathway. This enhances the accumulation of DNA strand breaks and promotes genomic instability and eventually leads to apoptosis. PARP catalyzes post-translational ADP-ribosylation of nuclear proteins that signal and recruit other proteins to repair damaged DNA and is activated by single-strand DNA breaks.
Drug Indication Treatment of metastatic castrate-resistant prostate cancer. Background: Senaparib is a novel, selective poly(ADP-ribose) polymerase-1/2 inhibitor with strong antitumor activity in preclinical studies. This first-in-human, phase 1, dose-escalation study examined the safety and preliminary efficacy of senaparib in patients with advanced solid tumors. Methods: Patients with advanced solid tumors were enrolled from three centers in Australia, using a conventional 3 + 3 design. Dose-escalation cohorts continued until the maximum tolerated dose or a recommended phase 2 dose was determined. Patients received one dose of oral senaparib and, if no dose-limiting toxicity occurred within 7 days, they received senaparib once daily in 3-week cycles. The primary end points were safety and tolerability. [1] Overall, senaparib was well tolerated in Australian patients with advanced, pretreated solid tumors and demonstrated preliminary evidence of antitumor activity. The current findings support further phase 2 and 3 investigations of senaparib in patients with solid tumors at the RP2D of 100 mg daily.[1] PARP inhibitors are promising anti-cancer agents with proven clinical activity based on mechanism of synthetic lethality. Senaparib (previously known as IMP4297) is a novel highly potent and selective oral PARP1/2 inhibitor with strong antitumor activity in preclinical studies. This first in human study investigated the tolerability, safety, PK, and preliminary antitumor activity of senaparib in Australia. Methods Adults with advanced, refractory solid tumours received senaparib orally QD, starting at 2mg. Dose escalation used a traditional 3+3 design and a modified Fibonacci sequence with 3-6 patients per cohort. DLT was evaluated in the first cycle. Dose expansion cohort enrolled patients with BRCA mutated (BRCA+) advanced solid tumors. Results As of Feb 25, 2020, 39 patients were enrolled in 10 dose levels (2 to 150mg). No DLTs were observed. The most frequent treatment emergent adverse events (TEAE) were headache (25.6%), fatigue (25.6%), constipation (17.9%), diarrhea (15.4%), nausea (12.8%), vomiting (12.8%) and anemia (10.3%). Treatment-related adverse events (TRAE) were observed in 8 (21%) patients starting from 40mg dose group. The most frequent TRAEs were nausea (8%), thrombocytopenia (5%) and fatigue (5%). An event of grade 4 thrombopenia in 80mg was the only serious TRAE. Four (10%) patients interrupted and 6 (15%) patients discontinued therapy due to AEs. The overall ORR and DCR was 15% and 85% respectively. In 8 evaluable ovarian cancer patients, ORR was 38% and DCR was 75%. A prolonged (> 20 months) PR response was observed in one BRCA+ ovarian cancer patient and a > 50% decrease of PSA for 11 months was observed in one BRCA- prostate cancer patients. The plasma exposure increased proportionally with doses ranging from 2mg to 80mg and became nonlinear ranging from 80mg to150mg cohorts. Conclusions Senaparib demonstrated encouraging clinical benefit and a favorable tolerability profile in patients with advanced solid tumour. The 100 mg orally QD was selected as the RP2D in Australia based on safety, pharmacokinetics and clinical activity. Clinical trial information: NCT03507543. [2] |
| 分子式 |
C24H20F2N6O3
|
|---|---|
| 分子量 |
478.450811386108
|
| 精确质量 |
478.16
|
| 元素分析 |
C, 60.25; H, 4.21; F, 7.94; N, 17.57; O, 10.03
|
| CAS号 |
1401682-78-7
|
| 相关CAS号 |
1401682-78-7; 1401683-39-3 (HCl)
|
| PubChem CID |
68389008
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
2
|
| tPSA |
98.7
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
804
|
| 定义原子立体中心数目 |
0
|
| SMILES |
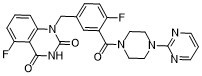
FC1=CC=C(C=C1C(N1CCN(C2N=CC=CN=2)CC1)=O)CN1C(NC(C2C(=CC=CC1=2)F)=O)=O
|
| InChi Key |
VBTUJTGLLREMNW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H20F2N6O3/c25-17-6-5-15(14-32-19-4-1-3-18(26)20(19)21(33)29-24(32)35)13-16(17)22(34)30-9-11-31(12-10-30)23-27-7-2-8-28-23/h1-8,13H,9-12,14H2,(H,29,33,35)
|
| 化学名 |
5-fluoro-1-[[4-fluoro-3-(4-pyrimidin-2-ylpiperazine-1-carbonyl)phenyl]methyl]quinazoline-2,4-dione
|
| 别名 |
IMP4297; IMP-4297; Senaparib; 1401682-78-7; Senaparib [INN]; MNZ4OP95CF; UNII-MNZ4OP95CF; 5-fluoro-1-[[4-fluoro-3-(4-pyrimidin-2-ylpiperazine-1-carbonyl)phenyl]methyl]quinazoline-2,4-dione; IMP 4297; Senaparib
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~83.3 mg/mL (~174.2 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (4.35 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.35 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0901 mL | 10.4504 mL | 20.9008 mL | |
| 5 mM | 0.4180 mL | 2.0901 mL | 4.1802 mL | |
| 10 mM | 0.2090 mL | 1.0450 mL | 2.0901 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04822961 | Not yet recruiting | Drug: Placebo Drug: Senaparib |
mCRPC | Impact Therapeutics, Inc. | December 31, 2021 | Phase 2 |
| NCT04434482 | Recruiting | Drug: IMP4297 (senaparib) |
Advanced Solid Tumours Small Cell Lung Cancer |
Impact Therapeutics, Inc. | August 7, 2020 | Phase 1 Phase 2 |
| NCT05269316 | Recruiting | Drug: IMP9064 | Advanced Solid Tumor Solid Tumor |
Impact Therapeutics, Inc. | February 11, 2022 | Phase 1 |