| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
HCV NS3/4A protease (Ki = 0.36 nM); HCV replication (EC50 = 7.8 nM); SARS-CoV-2 Mpro (IC50 = 9.6±2.3 μM); SARS-CoV-2 RdRp (IC50 = 5.5±0.2 μM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:simeprevir (TMC435350) 在 Huh7-Luc 细胞中的抗病毒活性呈剂量依赖性,TMC435350 测定的 EC50 和 EC90 值分别为 8 nM 和 24 nM。 TMC435350 对 NS3/4A 蛋白酶的抑制具有时间依赖性,基因型 1a 的总体 Kis 估计为 0.5 nM,基因型 1b 的总体 Kis 估计为 0.4 nM。 TMC435350 是 HCV NS3/4A 蛋白酶 (Ki=0.36 nM) 和病毒复制(复制子 EC50=7.8 nM)的有效抑制剂。激酶测定:使用荧光共振能量转移裂解测定法测定 simeprevir 对 NS3/4A 的体外抑制活性,其中 RetS1 肽底物源自基因型 1a NS4A-4B 连接,以及细菌表达的全长 NS3 蛋白酶结构域,补充了 NS4A 肽。简而言之,NS3/4A 在 TMC435350 存在下预孵育 10 分钟,然后添加 RetS1 底物并连续测量荧光 20 分钟(激发,355 nm;发射,500 nm)。底物的裂解表示为用载体对照观察到的裂解的百分比。细胞测定:Huh7-Luc 细胞以 2,500 个细胞/孔的密度接种在 384 孔板中的 Dulbeccos 改良 Eagles 培养基加 10% 胎牛血清中,并与一系列浓度的连续稀释的 simeprevir (TMC435350) 一起孵育,在没有 G418 的情况下,最终 DMSO 浓度为 0.5%。孵育 72 小时后,将 Steady Lite 试剂以 1:1 的比例添加到培养基中,并使用 ViewLux 读数器测量荧光素酶信号。
|
| 体内研究 (In Vivo) |
在大鼠中,TMC435350(40 mg/kg,po)广泛分布于肝脏和肠道(浓度-时间曲线比>35下的组织/血浆面积),绝对生物利用度为44%。
药理学研究[1] 吸收[1] Simeprevir的吸收期相对较长,在4-6小时后达到最大浓度(Cmax)。连续5天多次给药后,Cmax和24小时后的血浆浓度-时间曲线下面积(AUC24h)在75mg至200mg之间按剂量比例增加,表明首过代谢和/或外排转运蛋白饱和。200mg每日一次剂量组的24小时AUC约为100mg每日一次的10倍。每天一次给药7天后达到稳态。与肝功能正常的HCV未感染受试者相比,Child-Pugh B和C肝硬化患者中未感染HCV的受试者暴露于西普韦的剂量分别高出2.4倍和5.2倍。与单独给药相比,在与PEG-IFN-α和RBV联合给药期间,西莫普韦的血浆Cmax和AUC24h相似。在HCV感染的受试者中,平均稳态血浆浓度为1936 ng/mL,比之前体外研究中确定的EC50值高出200多倍。虽然给药后24小时血浆暴露量降至EC50左右,但给药后31小时内,肝脏浓度仍高于复制子99%有效浓度(EC99),因此表明每日一次给药的可行性。此外,在一项I期研究中,研究表明,健康的日本志愿者中西梅普韦的暴露量高于高加索志愿者。在III期试验中,亚洲受试者(n=14)的平均血浆西梅普韦暴露量是该试验普通人群的3.4倍。当与食物一起给药时,西美普韦的AUC24h增加了61%-69%;因此,西莫普韦应与食物一起服用。最后,simeprevir是P-糖蛋白的底物和抑制剂。21 分布[1] Simeprevir广泛(99.9%)结合血浆蛋白,主要是白蛋白。单次口服给药后,绝对生物利用度为44%。进入人肝细胞的转运被认为是由OATP1B1/3介导的。在大鼠中,发现肝脏与血液的比例为29:1,这意味着肝脏分布良好。对于人类,在临床前研究中,肝脏与血浆的浓度比很高(比率为39)。小肠的组织/血浆AUC比值最高(128)。虽然组织中的西莫普韦浓度在给药后4小时内达到峰值,但肝脏中的西马普韦浓度仍高于EC99达31小时,血浆浓度在给药剂后8小时高于EC99,在24小时左右高于EC50。 代谢[1] Simeprevir与telaprevir一样,在较小程度上与boceprevir一样,由CYP3A4代谢。因此,它可能与CYP3A酶的中度或重度抑制剂和诱导剂发生药物相互作用,同时西咪替韦的暴露量显著增加或减少。与博切普韦和特拉匹韦不同,西梅普韦是一种肠道细胞色素3A4的抑制剂,但不是肝脏CYP3A4。评估了低剂量(600mg)强效CYP3A诱导剂利福平对西梅普韦药代动力学的影响,结果表明,利福平和西梅普韦可使AUC24h降低48%,而Cmax增加31%。尽管中度肝损伤受试者的西美普韦暴露量高于健康受试者,但中度肝损伤患者不需要调整剂量。 排泄[1] Simeprevir通过胆汁排泄消除。单次服用200mg西美普韦后,粪便中回收了约91%的总放射性,尿液中回收了不到1%的放射性,这表明西美普韦可通过胆汁排泄从体内排出,而肾脏排泄则无关紧要。HCV感染患者的消除半衰期为41小时,几乎是HCV未感染者的3-4倍。西咪替韦的药代动力学参数也不受肾功能的影响,轻度、中度或重度肾功能损害的患者无需调整剂量。然而,尚未对终末期肾病患者或血液透析患者的安全性和有效性进行研究。 药效学[1] 西咪替韦剂量为75mg每日一次(QD)或以上时,西咪替韦暴露量与抗病毒活性之间没有明确的药代动力学/药效学关系。在III期试验中,在西美普韦的暴露范围内,没有观察到明显的疗效暴露-反应关系(快速病毒学反应[RVR]、SVR、病毒突破[VBT]或复发)。在西莫普韦的临床试验中,西莫普韦暴露量增加与不良反应频率增加有关,包括皮疹和光敏性。 |
| 酶活实验 |
使用源自基因型 1a NS4A-4B 连接的 RetS1 肽底物和细菌表达的全长 NS3 蛋白酶结构域(补充有 NS4A 肽)的荧光共振能量转移裂解测定,观察了 simeprevir 对 NS3/ 的体外抑制活性。 4A已确定。总之,在 TMC435350 存在下预孵育 10 分钟后,将 RetS1 底物添加到 NS3/4A 中。然后连续监测荧光20分钟(激发波长:355 nm;发射波长:500 nm)。基质裂解报告为在载体对照中观察到的裂解的百分比。
生化蛋白酶测定。[2] 在基于FRET的连续检测中监测HCV基因型1至6 NS3蛋白的蛋白酶活性。测定缓冲液含有25μM NS4A肽、50 mM Tris-HCl(pH 7.5)、15%甘油(体积比)、0.6 mM月桂基二甲胺N-氧化物和10 mM二硫苏糖醇。该缓冲液是通过在4°C下储存的其他成分的混合物中加入NS4A肽和二硫苏糖醇每天新鲜制备的。最终测定条件还包括0.5μM FRET底物、规定的可变浓度NS3/4A和ITMN-8187,以及高达5%的二甲亚砜(DMSO)(通过添加抑制剂或模拟处理)。在室温下在黑色96孔板中进行测定,并使用SpectraMax M5或SpectraMax EM板读数器(Molecular Devices,Sunnyvale,CA)收集荧光数据,激发和发射波长分别设置为490和520 nm。 使用NS3引发的反应(不预先培养酶和抑制剂)测定ITMN-8187对NS3/4A抑制的半最大抑制浓度(IC50)。除1a和3a(分别为0.1 nM和0.4 nM)外,所有测试的基因型变体的NS3最终1倍测定浓度均为0.05 nM。通过按指定顺序加入并混合170μl 1×测定缓冲液、10μl 20×ITMN-8187的DMSO(或DMSO空白)溶液、10μl20×底物和10μl的20×NS3/4A原液来制备测定孔。在加入NS3/4A并混合后立即开始1小时的数据采集。根据前30分钟的进展曲线斜率计算反应速率。将剂量反应曲线(速率与ITMN-8187的log10浓度)拟合到4参数逻辑函数中,以提取IC50。 细胞色素P450(CYP)抑制和诱导试验。[2] 通过体外评估CYP抑制来研究ITMN-8187的药物相互作用潜力,以评估人肝微粒体中CYP酶(CYP1A2、CYP2C8、CYP2C9、CYP2C19、CYP2D6和CYP3A)的IC50和时间依赖性抑制潜力(XenoTech股份有限公司,见补充方法)。简而言之,化合物与人肝微粒体、CYP探针底物和NADP氧化酶(NADPH)一起孵育。通过加入乙腈进行蛋白质沉淀来终止反应。离心后,通过LC-MS/MS分析上清液,以量化每种CYP酶的探针底物的特定代谢物形成程度。使用选择性探针底物在新鲜人肝细胞中研究了ITMN-8187诱导CYP酶活性(CYP1A2、CYP2B6和CYP3A4)的潜力。 |
| 细胞实验 |
使用补充有 10% 胎牛血清的 Dulbecco 改良 Eagles 培养基,将 Huh7-Luc 细胞以 2,500 个细胞/孔的密度接种在 384 孔板中。然后将细胞与不同浓度的连续稀释的 simeprevir (TMC435350) 一起培养,在不存在 G418 的情况下,最终 DMSO 浓度为 0.5%。经过 72 小时的孵育期后,将 Steady Lite 试剂以 1:1 的比例添加到培养基中后,使用 ViewLux 读数器测量荧光素酶信号。
HCV复制子检测。[2] Huh7-luc/neo-ET细胞和pFK I389luc-ubi-neo/NS3-3′/ET HCV复制子已从Reblikon GMBH获得许可。稳定的HCV复制子(pFK I38.luc-ubineo/NS3-3′/ET)表达萤火虫荧光素酶泛素-新霉素磷酸转移酶融合蛋白。含有三种细胞培养适应性突变(E1202G、T1280I、K1846T)的NS3/5B HCV多聚蛋白的表达是由脑心肌炎病毒内部核糖体进入位点驱动的。InterMune产生的HCV基因型1a亚基因组复制子代表化合物结合位点远端NS3 N末端附近的1b-1a嵌合体。含有亚基因组HCV复制子的Huh7细胞在37°C下在含有10%热灭活FBS、2 mM l-谷氨酰胺、1%非必需氨基酸、50 IU/ml青霉素、50μg/ml链霉素溶液和0.5 mg/ml G418的DMEM中的5%CO2中培养。将含有HCV复制子的Huh7细胞以5×103个细胞/孔的密度铺在含有100μl DMEM和G418的96孔组织培养板上。大约24小时后,取出培养基,用90μl缺乏G418的DMEM代替。将ITMN-8187或测试化合物在DMSO中连续稀释3倍,分为两行,每次测定半最大有效浓度(EC50)。将连续稀释的化合物溶液在缺乏血清和G418的DMEM中稀释10倍;将10μl这些化合物在培养基中的溶液加入到复制的组织培养板中。最终体积为100μl,DMSO浓度为1%。将培养板在37°C下孵育约48小时。48小时后,对组织培养板进行显微镜目视检查,以评估化合物的溶解度。从两个复制板中的一个中取出培养基,使用Bright Glo萤光素酶测定法测量萤光素酶活性以确定EC50s。ATPlite检测试剂盒用于测定第二板中细胞和培养基的ATP水平,以测定半最大细胞毒性浓度(CC50s)。Bright Glo和ATPlite检测试剂盒均按照制造商的说明使用。类似地测定了测试化合物对R155K、A156T和D168V HCV 1b突变复制子的效力水平。为了评估血清浓度对化合物效力的影响,在40%FBS存在的情况下进行了上述测定。血清的影响表示为相对于10%FBS参考条件的倍数偏移。 |
| 动物实验 |
Sprague-Dawley (SD) rats and cynomolgus monkeys
3 mg/kg Oral administration In vivo preclinical studies.[2] Pharmacokinetic properties of ITMN-8187 and simpeprevir were evaluated in Sprague-Dawley (SD) rats, beagle dogs, and cynomolgus monkeys. Procedures were performed under protocols approved by the Institutional Animal Care and Use Committee of the test facility. The animals were fasted overnight and through 4 h after administration of ITMN-8187. SD rats, beagle dogs, and cynomolgus monkeys (three males per species per dosing route) were administered with ITMN-8187 at 3 mg/kg by oral gavage or 0.5 mg/kg by intravenous (i.v.) bolus injection. The formulations used in the i.v. and oral studies were 0.5-mg/ml and 0.6-mg/ml solutions, respectively, in DMSO-solutol-saline (2/2/96, vol/vol/vol). For each species, blood samples were collected at 0.25, 0.5, 1, 2, 4, 8, 12, and 24 h after both i.v. and oral administration. Blood samples were also collected at 5 min after dosing from i.v. studies in all three species. Blood samples were collected in tubes containing tripotassium EDTA (K3EDTA), processed for plasma by centrifugation at 5°C, and stored at −20°C until analysis was performed. In addition, following oral administration of ITMN-8187 at 3 mg/kg, liver tissues were collected from rats and monkeys at predefined time points, rinsed in phosphate-buffered saline, dried, and stored in vials at −80°C until analysis. Drug concentration from each plasma or liver tissue sample at each time point was individually quantified by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Pharmacokinetic analysis of the concentration in plasma-time profiles was performed using Phoenix/WinNonlin version 6.3. HCV chimeric mouse model.[2] Chimeric mice were created by transplanting human hepatocytes into immunodeficient transgenic mice carrying Alb-uPA. The immunodeficient homozygous Alb-uPA mice carrying chimeric mouse/human livers with serum were then infected with virus from patients with HCV infection; the inoculum varied by HCV genotype or mutations. The HCV chimeric mouse model used in these studies was provided by Phoenix Bio. Briefly, male uPA+/+-SCID mice 2 to 4 weeks of age were transplanted with human hepatocytes with an estimated replacement index of ≥70%, based on human albumin measurements (>9 mg/ml). After transplantation, the mice were infected at 10 to 14 weeks with HCV genotypes 1a or 1b by inoculation with HCV-positive patient serum. HCV infection in the chimeric mice was confirmed by the presence of serum HCV RNA measurements by real-time PCR (RT-PCR; lower limit of quantification, 4 × 104 copies/ml). uPA+/+-SCID mice confirmed to be infected with HCV genotypes 1a or 1b were dosed for 4 days with vehicle (2% Solutol) or ITMN-8187 at 30 mg/kg once daily by oral gavage. The vehicle and ITMN-8187 dosing solutions were formulated fresh daily. Serum was collected from individual mice on day 0 before treatment with vehicle or ITMN-8187 began (baseline), at 6 and 12 h postdose with vehicle or ITMN-8187 on day 1, and then once daily on days 2 to 4 for measurement of HCV RNA concentration. Data are expressed as the average HCV RNA concentration (copies/milliliter) per experimental group (n = 4); statistical differences (P < 0.05) between the vehicle-treated control mice and ITMN-8187-treated groups were analyzed using the Student t test. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The mean absolute bioavailability of simeprevir following a single oral 150 mg dose of simeprevir capsule in fed conditions is 62%. Maximum plasma concentrations (Cmax) are typically achieved between 4 to 6 hours following the oral administration. Simeprevir is predominantly eliminated through biliary excretion. In a radioactivity study, 91% of radiolabeled drug was detected in the feces and less than 1% was detected in the urine. From the recovered drug in the feces, the unchanged form of simeprevir accounted for 31% of the total administered dose. The volume of distribution for simeprevir has yet to be determined. In animal studies, simeprevir is extensively distributed to gut and liver (liver:blood ratio of 29:1 in rat) tissues. The clearance of simeprevir has yet to be determined. Simeprevir is extensively bound to plasma proteins (greater than 99.9%), primarily to albumin and, to a lesser extent, alfa 1-acid glycoprotein. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment. Administration of simeprevir with food to healthy subjects increased the relative bioavailability (AUC) by 61% and 69% after a high-fat, high-caloric (928 kcal) and normal-caloric (533 kcal) breakfast, respectively, and delayed the absorption by 1 hour and 1.5 hours, respectively. Due to increased bioavailability, Olysio should be administered with food. The type of food does not affect exposure to simeprevir. Elimination of simeprevir occurs via biliary excretion. Renal clearance plays an insignificant role in its elimination. Following a single oral administration of 200 mg (14)C-simeprevir to healthy subjects, on average 91% of the total radioactivity was recovered in feces. Less than 1% of the administered dose was recovered in urine. Unchanged simeprevir in feces accounted for on average 31% of the administered dose. In animals, simeprevir is extensively distributed to gut and liver (liver:blood ratio of 29:1 in rat) tissues. In vitro data and physiologically-based pharmacokinetic modeling and simulations indicate that hepatic uptake in humans is mediated by OATP1B1/3. For more Absorption, Distribution and Excretion (Complete) data for Simeprevir (10 total), please visit the HSDB record page. Metabolism / Metabolites Simeprevir undergoes hepatic metabolism. The primary metabolic pathway involves CYP3A system-mediated oxidation. Involvement of CYP2C8 and CYP2C19 cannot be excluded. Following a single oral administration of 200 mg (1.3 times the recommended dosage) (14)C-simeprevir to healthy subjects, the majority of the radioactivity in plasma (mean: 83%) was accounted for by unchanged drug and a small part of the radioactivity in plasma was related to metabolites (none being major metabolites). Metabolites identified in feces were formed via oxidation at the macrocyclic moiety or aromatic moiety or both and by O-demethylation followed by oxidation. Simeprevir is metabolized in the liver. In vitro experiments with human liver microsomes indicated that simeprevir primarily undergoes oxidative metabolism by the hepatic CYP3A system. Involvement of CYP2C8 and CYP2C19 cannot be excluded. Co-administration of Olysio with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir, and co-administration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir. The in vitro metabolism of 14C-TMC435 was investigated in hepatocytes and liver microsomes of mouse, rat, rabbit, monkey and human. The metabolic activity reported in vitro from animals and man was low. Phase II conjugation pathways of Phase I metabolites were formed in hepatocytes. Parent TMC435 was found in much greater levels than any metabolite in vitro. More than 20 metabolites were identified. The metabolic Phase I route of highest importance were O-demethylation of unchanged drug (particularly in animals), oxidation of unchanged drug and oxidized metabolites (particularly in monkey and man) and glucuronidation was the major Phase II of oxidized metabolites (less in human). Only one human metabolite identified in vitro not seen in rat or dog was M22 (oxidized unchanged drug) but this metabolite was identified in rat (feces). In vivo data reveals that the main moiety present in plasma of rat, dog and man was parent TMC435. The major metabolites reported in vivo in plasma from animals and human were M18 and M21. O-desmethyl-TMC435 M21 was the only common circulating metabolite found in rat dog and human plasma (M21: 8% of the mean TMC435 plasma and only small traces in dogs), while M18 was common to plasma of rats and dogs but with respect to the parent compound they appeared with low concentrations (M18: between 28.9% and 12.5% in rats, with only small traces in dogs). Only traces of metabolites M18, M21 and M8 formed by O-demethylation and oxidation at the aromatic moiety were reported in dog plasma. M21 represents less than 10% of unchanged drug and also total radioactivity therefore systemic exposure to M21 was not assessed in the safety evaluation studies. M21 did not appear to accumulate in man. In bile from rats, moderately high levels of parent compound were reported (0.11 to 17.2%). TMC435 metabolites in this matrix were formed mainly by hydroxylation and O-demethylation and also by glucuronidation. The most important metabolic route TMC435 in rat and dog was O-demethylation of the parent drug to M18 (12.8%- 6.4% male-female rats; 18.8% dogs). In rats other metabolites were formed by oxidation of M18 and oxidation of unchanged drug. In dogs, further oxidation of M18 to M14 and M8, and of the unchanged drug to M21, M16 and M11 were also reported as minor routes. The human metabolism profile suggests that TMC435 is mainly metabolized by two main routes, (1) oxidation of unchanged drug, either at the macrocyclic moiety (M27, M21 and M22), or at the aromatic moiety (M26 and M16), or both (M23, M24, M25 and M11) and (2) the O-demethylation of unchanged drug to M18, followed by oxidation on the macrocyclic moiety to M14 and by oxidation on the aromatic moiety to M5, appears to be the secondary metabolic pathway in man. M21 and M22 were the most important metabolites in human faeces. Other relevant metabolites (1% of the dose) were M11, M16, M27 and M18. All metabolites detected in human feces were detected in vitro and/or in vivo in rat and/or dog feces. The main CYP enzymes involved in TMC435 metabolism were CYP3A enzymes although in vitro data suggests the involvement of CYP2C8 and CYP2C19. Biological Half-Life The elimination half-life of simeprevir following 200mg dose administration is about 41 hours in HCV-positive patients and 10 to 13 hours in individuals without HCV infection. The half-life was variable among species accounting to 4.0 hr in rats, 3.7 hr in rabbits and dogs and 5 to 6 hr in Rhesus and Cynomolgus monkeys. The terminal elimination half-life of simeprevir was 10 to 13 hours in hepatitis C virus (HCV)-uninfected subjects and 41 hours in HCV-infected subjects receiving 200 mg (1.3 times the recommended dosage) of simeprevir. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Simeprevir is a white to almost white powder. Simeprevir is used in conjunction with peginterferon alfa and ribavirin for the treatment of chronic hepatitis C virus (HCV) genotype 1 infection in adults with compensated liver disease (including cirrhosis) who are treatment-naive (previously untreated) or in whom prior treatment with interferon and ribavirin failed (including those with prior null response, prior partial response, or prior relapse). Simeprevir must be used in conjunction with peginterferon alfa (peginterferon alfa-2a or peginterferon alfa-2b) and ribavirin and should not be used alone for the treatment of chronic HCV infection. HUMAN EXPOSURE AND TOXICITY: Very few data are available on the effects of overdose to simeprevir. Simeprevir was generally well tolerated when given as single doses up to 600 mg or once daily doses up to 400 mg for 5 days in healthy adult subjects, and as 200 mg once daily for 4 weeks in adult patients with HCV. ANIMAL STUDIES: Simeprevir was well tolerated after single doses up to 500 mg/kg in mice, 1000 mg/kg in rats, 160 mg/kg in dogs and 300 mg/kg in monkeys. There were no adverse effects of simeprevir on vital functions (cardiac, respiratory and central nervous system) in animal studies. Repeat dose oral toxicity studies with simeprevir were conducted in mice (up to 3 months), rats (up to 6 months), dogs (up to 9 months), and monkeys (up to 28 days). Gastrointestinal effects were observed in all species. A higher incidence of soft, mucoid or pale feces was seen in mice, rats and/or dogs. The presence of swelling/vacuolization of apical enterocytes in the duodenum and jejunum was noted in mice, rats and dogs. The compound formulation caused abnormal stomach contents and/or abdominal distention, in mice and rats, as a result of delayed gastric emptying. Liver effects were observed in mice, rats and dogs. These findings were often accompanied by increases in bilirubin, and liver enzymes in plasma. In a mouse embryofetal study at doses up to 1000 mg/kg, simeprevir resulted in early and late in utero fetal losses and early maternal deaths at an exposure approximately 6 times higher than the mean AUC in humans at the recommended 150 mg daily dose. Significantly decreased fetal weights and an increase in fetal skeletal variations were seen at exposures approximately 4 times higher than the mean AUC in humans at the recommended daily dose. In a rat pre- and postnatal study, maternal animals were exposed to simeprevir during gestation and lactation at doses up to 1000 mg/kg/day. In pregnant rats, simeprevir resulted in early deaths at 1000 mg/kg/day corresponding to exposures similar to the mean AUC in humans at the recommended 150 mg once daily dose. Significant reduction in body weight gain was seen at an exposure 0.7 times the mean AUC in humans at the recommended 150 mg once daily dose. The developing rat offspring exhibited significantly decreased body weight and negative effects on physical growth (delay and small size) and development (decreased motor activity) following simeprevir exposure in utero (via maternal dosing) and during lactation (via maternal milk to nursing pups) at a maternal exposure similar to the mean AUC in humans at the recommended 150 mg once daily dose. Subsequent survival, behavior and reproductive capacity were not affected. In a rat fertility study at doses up to 500 mg/kg/day, 3 male rats treated with simeprevir (2/24 rats at 50 mg/kg/day and 1/24 rats at 500 mg/kg/day) showed no motile sperm, small testes and epididymides, and resulted in infertility in 2 out of 3 of the male rats at approximately 0.2 times the mean AUC in humans. Simeprevir was not genotoxic in a series of in vitro and in vivo tests including the Ames test, the mammalian forward mutation assay in mouse lymphoma cells or the in vivo mammalian micronucleus test. Interactions In vitro, simeprevir is a substrate and inhibitor of P-glycoprotein (P-gp) transport. Concomitant use of simeprevir with drugs that are P-gp substrates may result in increased concentrations of such drugs. Pharmacokinetic interaction with cyclosporine (increased cyclosporine concentrations). Cyclosporine dosage adjustments are not needed when used concomitantly with simeprevir; routine monitoring of cyclosporine concentrations is recommended. Concomitant use of simvastatin (single 40-mg dose) and simeprevir (150 mg once daily for 10 days) resulted in a 1.5-fold increase in simvastatin AUC due to inhibition of OATP1B1 and/or CYP3A4 by simeprevir. If simvastatin is used concomitantly with simeprevir, dosage of simvastatin should be titrated carefully and the lowest necessary dosage of simvastatin used; the patient should be monitored for safety. Concomitant use of a rosuvastatin (single 10 mg dose) and simeprevir (150 mg once daily for 7 days) resulted in a 2.8-fold increase in rosuvastatin AUC due to inhibition of OATP1B1 by simeprevir. If rosuvastatin is used concomitantly with simeprevir, dosage of rosuvastatin should be initiated at 5 mg once daily and should not exceed 10 mg once daily. For more Interactions (Complete) data for Simeprevir (38 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antiviral Agents; Protease Inhibitors Olysio is a hepatitis C virus (HCV) NS3/4A protease inhibitor indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection as a component of a combination antiviral treatment regimen. /Included in US product label/ Olysio monotherapy is not recommended. Olysio combination with peginterferon alfa and ribavirin: screening patients with hepatitis C virus (HCV) genotype 1a infection for the presence of virus with the NS3 Q80K polymorphism is strongly recommended and alternative therapy should be considered if HCV genotype 1a with Q80K is detected. Olysio is not recommended in patients who have previously failed therapy with a treatment regimen that included Olysio or other hepatitis C virus (HCV) protease inhibitors. Drug Warnings Simeprevir contains a sulfonamide moiety. In clinical trials of simeprevir, an increased incidence of rash or photosensitivity was not observed in the 16 patients who had a history of sulfa allergy. However, data are insufficient to exclude an association between sulfa allergy and the frequency or severity of adverse reactions reported with simeprevir. During the 12 weeks of treatment with Olysio, dyspnea was reported in 12% of Olysio-treated subjects compared to 8% of placebo-treated subjects (all grades; pooled Phase 3 trials). All dyspnea events reported in Olysio-treated subjects were of mild or moderate severity (Grade 1 or 2). There were no Grade 3 or 4 dyspnea events reported and no subjects discontinued treatment with Olysio due to dyspnea. Sixty-one percent (61%) of dyspnea events occurred in the first 4 weeks of treatment with Olysio. Adverse effects reported in more than 20% of patients receiving simeprevir in conjunction with peginterferon alfa and ribavirin in clinical trials and occurring with an incidence at least 3% higher than that reported in patients receiving placebo in conjunction with peginterferon alfa and ribavirin include rash (including photosensitivity), pruritus, and nausea. Rash has been reported in patients receiving simeprevir in conjunction with peginterferon alfa and ribavirin. Rash occurred most frequently during the first 4 weeks of treatment, but can occur at any time during the course of treatment. Rash generally was mild or moderate in severity, but severe rash and rash requiring discontinuance of the drug have been reported. Patients with mild to moderate rash should be monitored for possible progression (e.g., development of oral lesions, conjunctivitis, systemic symptoms). If rash becomes severe, simeprevir should be discontinued. Patients should be monitored until rash resolves. For more Drug Warnings (Complete) data for Simeprevir (12 total), please visit the HSDB record page. Pharmacodynamics Simeprevir is a direct-acting antiviral agent and inhibitor for HCV NS3/4A protease, which is an important enzyme required for viral replication. Unlike [DB08873] and [DB05521], simeprevir is a competitive, reversible, macrocyclic, noncovalent inhibitor. The macromolecular cyclic portion of the molecule improves the affnity and selectivity characteristics, which allows rapid association and slow dissociation to the protein target through noncovalent binding. Simeprevir (TMC435, Olysio™), a second-generation hepatitis C virus (HCV) protease inhibitor, has been recently approved for the treatment of genotype 1 chronic hepatitis C in combination with pegylated interferon and ribavirin. This molecule has very different characteristics from first-generation protease inhibitors. Results from trials show that simeprevir is highly effective and safe, with few adverse events. We discuss the specific features of this new treatment option for HCV infection, in terms of in vitro data, pharmacological data, and clinical trials. We also discuss the impact of Q80K polymorphism at baseline. Studies evaluating interferon-free regimens with simeprevir are ongoing. Future combinations of two or more direct-acting antiviral agents, targeting different viral enzymes and with synergistic antiviral effects, will be approved, allowing treatment of pan-genotypic HCV with optimized sustained virologic responses. Simeprevir will undoubtedly be part of future treatment strategies.[1] The current paradigm for the treatment of chronic hepatitis C virus (HCV) infection involves combinations of agents that act directly on steps of the HCV life cycle. Here we report the preclinical characteristics of ITMN-8187, a nonmacrocyclic inhibitor of the NS3/4A HCV protease. X-ray crystallographic studies of ITMN-8187 and simeprevir binding to NS3/4A protease demonstrated good agreement between structures. Low nanomolar biochemical potency was maintained against NS3/4A derived from HCV genotypes 1, 2b, 4, 5, and 6. In cell-based potency assays, half-maximal reduction of genotype 1a and 1b HCV replicon RNA was afforded by 11 and 4 nM doses of ITMN-8187, respectively. Combinations of ITMN-8187 with other directly acting antiviral agents in vitro displayed additive antiviral efficacy. A 30-mg/kg of body weight dose of ITMN-8187 administered for 4 days yielded significant viral load reductions through day 5 in a chimeric mouse model of HCV. A 3-mg/kg oral dose administered to rats, dogs, or monkeys yielded concentrations in plasma 16 h after dosing that exceeded the half-maximal effective concentration of ITMN-8187. Human microdose pharmacokinetics showed low intersubject variability and prolonged oral absorption with first-order elimination kinetics compatible with once-daily dosing. These preclinical characteristics compare favorably with those of other NS3/4A inhibitors approved for the treatment of chronic HCV infection.[2] |
| 分子式 |
C38H47N5O7S2
|
|
|---|---|---|
| 分子量 |
749.94
|
|
| 精确质量 |
749.291
|
|
| 元素分析 |
C, 60.86; H, 6.32; N, 9.34; O, 14.93; S, 8.55
|
|
| CAS号 |
923604-59-5
|
|
| 相关CAS号 |
Simeprevir sodium;1241946-89-3;Simeprevir-13C,d3
|
|
| PubChem CID |
24873435
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 折射率 |
1.653
|
|
| LogP |
4.99
|
|
| tPSA |
193.51
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
10
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
52
|
|
| 分子复杂度/Complexity |
1490
|
|
| 定义原子立体中心数目 |
5
|
|
| SMILES |
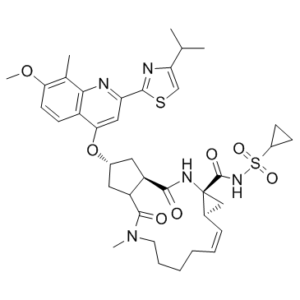
S(C1([H])C([H])([H])C1([H])[H])(N([H])C([C@@]12C([H])([H])[C@@]1([H])C([H])=C([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([C@]1([H])C([H])([H])[C@@]([H])(C([H])([H])[C@@]1([H])C(N2[H])=O)OC1=C([H])C(C2=NC(C([H])(C([H])([H])[H])C([H])([H])[H])=C([H])S2)=NC2C(C([H])([H])[H])=C(C([H])=C([H])C1=2)OC([H])([H])[H])=O)=O)(=O)=O |t:21|
|
|
| InChi Key |
JTZZSQYMACOLNN-VDWJNHBNSA-N
|
|
| InChi Code |
InChI=1S/C38H47N5O7S2/c1-21(2)30-20-51-35(40-30)29-18-32(26-13-14-31(49-5)22(3)33(26)39-29)50-24-16-27-28(17-24)36(45)43(4)15-9-7-6-8-10-23-19-38(23,41-34(27)44)37(46)42-52(47,48)25-11-12-25/h8,10,13-14,18,20-21,23-25,27-28H,6-7,9,11-12,15-17,19H2,1-5H3,(H,41,44)(H,42,46)/b10-8-/t23-,24-,27-,28-,38-/m1/s1
|
|
| 化学名 |
(1R,4R,6S,7Z,15R,17R)-N-cyclopropylsulfonyl-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (3.33 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.43 mg/mL (1.91 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 14.3 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.43 mg/mL (1.91 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3334 mL | 6.6672 mL | 13.3344 mL | |
| 5 mM | 0.2667 mL | 1.3334 mL | 2.6669 mL | |
| 10 mM | 0.1333 mL | 0.6667 mL | 1.3334 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Efficacy and Safety of Combinations of AL-335, Odalasvir (ODV) and Simeprevir (SMV) in the Treatment of Chronic Hepatitis C Infection
CTID: NCT02765490
Phase: Phase 2 Status: Completed
Date: 2019-11-20