| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
CDK9 (IC50 = 4 nM); CDK2 (IC50 = 38 nM); CDK7 (IC50 = 62 nM); CDK1 (IC50 = 480 nM); CDK4 (IC50 = 925 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:SNS-032 对 CDK1 和 CDK4 具有低敏感性,IC50 分别为 480 nM 和 925 nM。无论预后指标和治疗史如何,SNS-032都能在体外有效杀死慢性淋巴细胞白血病细胞。与 flavopiridol 和 roscovitine 相比,SNS-032 在抑制 RNA 合成和诱导细胞凋亡方面更有效。 SNS-032 的活性很容易可逆;去除 SNS-032 会重新激活 RNA 聚合酶 II,从而导致 Mcl-1 的重新合成和细胞存活。 SNS-032 抑制内皮细胞的三维毛细血管网络形成。 SNS-032 完全阻止 U87MG 细胞介导的 HUVEC 毛细血管形成。此外,SNS-032 显着阻止两种细胞系中 VEGF 的产生,SNS-032 阻止体外血管生成,这种作用可归因于 VEGF 的阻断。临床前研究表明,SNS-032 可诱导多种细胞系的细胞周期停滞和细胞凋亡。 SNS-032 通过抑制 CDK 2 和 7 来阻断细胞周期,并通过抑制 CDK 7 和 9 来阻断转录。SNS-032 活性不受人血清影响。 SNS-032 诱导膜联蛋白 V 染色和 caspase-3 激活呈剂量依赖性增加。在分子水平上,SNS-032 诱导 RNA 聚合酶 (RNA Pol) II 丝氨酸 2 和 5 的显着去磷酸化,并抑制 CDK2 和 CDK9 以及去磷酸化 CDK7 的表达。激酶测定:SNS-032 是 CDK2、CDK7 和 CDK9 的选择性抑制剂,IC50 分别为 38 nM、62 nM 和 4 nM。细胞测定:用 SNS-032 处理 6 或 24 小时的 CLL 细胞显示 RNA Pol II CTD 的 Ser2 和 Ser5 磷酸化降低,这似乎具有时间和浓度依赖性,并且在样品之间非常一致。对于Ser2的磷酸化,SNS-032的抑制大于Ser5的磷酸化,这与抑制CDK9的IC50低于抑制CDK7的IC50的事实相一致(4 nM vs 62 nM)纳米)。 SNS-032 暴露 6 小时后,CDK7 和 CDK9 的蛋白质水平稳定,但在 24 小时时下降。
|
| 体内研究 (In Vivo) |
SNS-032 在肿瘤共培养模型中阻止肿瘤细胞诱导的 VEGF 分泌。 SNS-032 是一种新型 CDK 抑制剂,具有更高的选择性和更低的细胞毒性,并已被证明可以延长实体瘤的稳定疾病。
SNS-032在体内改善肝功能和纤维化阶段[6] 雄性C57BL/6小鼠于周一、周三、周五腹腔注射2 ml·kg - 1 15% ccl4 -橄榄油,连续6周建立小鼠肝纤维化模型。注射3周后,小鼠分别接受2.5、5或10 mg·kg−1 SNS-032和5 mg·kg−1索拉非尼治疗3周(图1A)。然后采集各组大鼠血清和肝脏组织。我们发现实验组小鼠的肝脏重量和肝体重比与正常组相比有显著增加(p < 0.001)。使用2.5、5或10 mg·kg−1 SNS-032和5 mg·kg−1索拉非尼 治疗后,肝脏重量和肝/体重比显著降低 5和10 mg·kg−1 SNS-032治疗后,胶原沉积、炎症细胞浸润、肝纤维化程度显著降低(图1D)。半定量分析的天狼星红阳性面积和肝组织羟脯氨酸含量在实验组显著增加,在5、10 mg·kg−1 SNS-032和5 mg·kg−1索拉非尼组显著降低。实验组acta2阳性面积百分比显著增加,5、10 mg·kg−1 SNS-032和5 mg·kg−1索拉非尼组acta2阳性面积百分比下降。在生化特征方面,试验组血清ALT和AST水平显著升高,5、10 mg·kg−1 SNS-032和5 mg·kg−1索拉非尼组血清ALT和AST水平显著降低。 |
| 酶活实验 |
SNS-032 对 CDK2、CDK7 和 CDK9 表现出选择性,其 IC50 值分别为 38 nM、62 nM 和 4 nM。
内皮细胞的增殖、迁移和毛细血管网络的形成是血管生成的基本步骤,血管生成涉及到新血管的形成。本研究的目的是利用由人脐静脉内皮细胞(HUVECs)和人胶质母细胞瘤细胞(U87MG)组成的共培养系统,研究新型氨基噻唑SNS-032对体外血管生成关键步骤的影响。SNS-032是周期蛋白依赖性激酶2,7和9的有效选择性抑制剂,可抑制转录和细胞周期。在本研究中,我们检测了SNS-032存在下HUVECs和U87MG细胞的增殖和活力,并观察到两种细胞系对细胞增殖的剂量依赖性抑制。SNS-032抑制内皮细胞三维毛细血管网络的形成。在共培养研究中,SNS-032完全阻止了U87MG细胞介导的HUVECs毛细血管形成。该抑制剂在单独培养或与U87MG细胞共培养时也能阻止huvec的迁移。此外,SNS-032在两种细胞系中均能显著抑制血管内皮生长因子(VEGF)的产生,而在培养基中添加活性重组VEGF时,SNS-032对内皮细胞毛细血管网络形成和迁移的抑制作用较弱。综上所述,SNS-032可阻止体外血管生成,其作用可归因于阻断VEGF。[2] |
| 细胞实验 |
使用 Cell Titer-Glo (CTG) 发光测定法测量 HUVEC 和 U87MG 细胞的生长曲线。总共100毫升用于在96孔微孔板中接种U87MG细胞和HUVEC(2×103细胞/孔)。 24 小时标记后,将细胞暴露于不同浓度的 SNS-032 (0-0.5 mM) 24、48 或 72 小时。处理完成后,将 100 mL CTG 溶液添加到每个孔中,然后将孔在黑暗和室温下放置 20 分钟。将裂解液(50 mL)放入96孔白板中,并使用POLARstar OPTIMA测量发光。计算细胞生长百分比时,考虑添加 SNS-032 时的 100% 生长。
分析了SNS-032对HES和CML tki耐药细胞PDGFRα和Bcr-Abl信号通路、细胞凋亡和细胞周期的影响。在裸鼠模型中,通过移植BaF3-T674I、FIP1L1-PDGFRα和KBM5-T315I Bcr-Abl细胞来评估SNS-032的体内抗肿瘤活性。 结果:SNS-032抑制RNA聚合酶II Ser5和Ser2的磷酸化。SNS-032降低FIP1L1-PDGFRα和Bcr-Abl mRNA和蛋白水平,抑制表达FIP1L1-PDGFRα或Bcr-Abl的恶性细胞的增殖。它还降低了下游分子的磷酸化。它通过触发线粒体途径和死亡受体途径诱导细胞凋亡。 结论:该CDK7/9抑制剂能有效抑制fip1l1 - pdgfr α阳性HES细胞和bcr - abl阳性CML细胞,无论其对伊马替尼的敏感性如何。SNS-032可能具有通过消除癌基因依赖性来治疗血液恶性肿瘤的潜力[4]。 使用增殖和集落形成试验评估细胞毒性,Western blot分析评估靶调节,FACS分析评估细胞周期分布,RT-PCR评估转录抑制。 结果:SNS-032通过抑制cdk2和7来阻断细胞周期,通过抑制cdk7和9来阻断转录。RPMI-8226 MM细胞在300 nM (IC(90))下处理6小时足以导致细胞凋亡。这与cdk2,7和9的抑制有关,反映在底物信号分子中。SNS-032活性不受人血清影响。在治疗患者的PBMC中观察到靶调节。 结论:这些结果证明了SNS-032对CDKs 2、7和9的靶向调节,并确定了暴露6小时足以使RPMI-8226 MM细胞凋亡。结合SNS-032治疗的1期实体瘤患者PBMC靶标调节的证明,这些数据支持了SNS-032在MM和CLL中的临床研究。[3] |
| 动物实验 |
Simple nu/nu In barrier facilities with a 12-hour light-dark cycle, BALB/c mice are kept with unlimited access to food and water. On the flanks of 4- to 6-week-old male nude mice, a mixture of 1×107 BaF3-T674I cells with Matrigel or KBM5-T315I cells (3×107) is subcutaneously injected. Calipers are used every other day to measure tumors. The formula for calculating tumor volumes is a2×b×0.4, where a is the diameter that is the smallest and b is the diameter that is perpendicular to a. After the subcutaneous inoculation, mice are randomized to receive treatment with either vehicle (tissue culture medium containing 0.1% v/v) or SNS-032 (15 mg/kg injected intraperitoneally every two days) for approximately two weeks, or until the tumors are palpable (approximately 100 mm3). Prior to dilution, SNS-032 is dissolved in tissue culture grade DMSO. Each animal's body weight, eating habits, and level of motor activity are tracked as measures of overall health. Tumor xenografts are then promptly removed, weighed, stored, and fixed after the animals are put to sleep.
Forty-eight male C57BL6 mice (6-week-old, 18–20 g) were caged individually in a temperature- and humidity-controlled environment under a 12:12 light–dark cycle. Then, they were assigned into two groups randomly. The first group (control group, n = 8) was fed with normal diet and water freely. The second group (n = 40) was intraperitoneally injected with 2 ml·kg−1 15% carbon tetrachloride (CCl4)–olive oil on Mondays, Wednesdays, and Fridays for 3 weeks. Then, the mice in the second group were randomly allocated into five groups (experimental, low-dose SNS-032, medium-dose SNS-032, high-dose SNS-032, and sorafenib groups; n = 8). Subsequently, the mice in the low-, medium-, and high-dose SNS-032 and sorafenib groups were treated with 2.5, 5, and 10 mg·kg−1·day SNS-032 through intraperitoneal administration or 5 mg·kg−1·day sorafenib through intragastric administration for 3 weeks with the continuous injection of CCl4–olive oil. The CCl4 mouse model was used as the liver fibrosis model as previously described (Strnad et al., 2008).[6] |
| 参考文献 |
|
| 其他信息 |
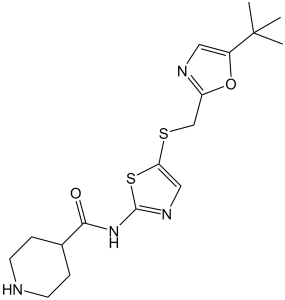
N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)piperidine-4-carboxamide is a secondary carboxamide resulting from the formal condensation of the carboxy group of piperidine-4-carboxylic acid with the amino group of 5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-amine. It is an ATP-competitive inhibitor of CDK2, CDK7 and CDK9 kinases and exhibits anti-cancer properties. It has a role as an apoptosis inducer, an antineoplastic agent, an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor and an angiogenesis inhibitor. It is a piperidinecarboxamide, a member of 1,3-oxazoles, a member of 1,3-thiazoles, an organic sulfide and a secondary carboxamide.
CDK Inhibitor SNS-032 is a small aminothiazole molecule and cyclin dependent kinase (CDK) inhibitor with potential antineoplastic activity. SNS-032 binds to and prevents the phosphorylation of cyclin-dependent kinases, especially CDK2, 7, and 9 that regulate cell cycle progression. Inhibition of CDKs leads to cell cycle arrest and induces apoptosis. As a result, this agent causes cytotoxicity and prevents further tumor cell growth. Drug Indication Investigated for use/treatment in cancer/tumors (unspecified), leukemia (unspecified), lymphoma (unspecified), multiple myeloma, and solid tumors. Mechanism of Action SNS-032 is a potent and selective inhibitor of CDKs 2, 7 and 9, which are critical in the communication and relay of signals to promote cellular growth and function. CDK2 is involved in cellular proliferation by regulating the initiation of and progression through the DNA-synthesis phase of the cell cycle. CDK7 and CDK9 are involved in transcriptional regulation of certain proteins involved in cell survival. Inappropriate activity by these CDKs can lead to unregulated proliferation, avoidance of apoptosis and increased cell survival, all of which are hallmarks of cancer. By selectively targeting these CDKs, SNS-032 may halt both aberrant cell proliferation and induce apoptosis. Inhibitors of cyclin-dependent kinases (Cdks) have been reported to have activities in chronic lymphocytic leukemia cells by inhibiting Cdk7 and Cdk9, which control transcription. Here we studied the novel Cdk inhibitor SNS-032, which exhibits potent and selective inhibitory activity against Cdk2, Cdk7, and Cdk9. We hypothesized that transient inhibition of transcription by SNS-032 would decrease antiapoptotic proteins, resulting in cell death. SNS-032 effectively killed chronic lymphocytic leukemia cells in vitro regardless of prognostic indicators and treatment history. This was associated with inhibition of phosphorylation of RNA polymerase II and inhibition of RNA synthesis. Consistent with the intrinsic turnover rates of their transcripts and proteins, antiapoptotic proteins, such as Mcl-1 and X-linked inhibitor of apoptosis protein (XIAP), were rapidly reduced on exposure to SNS-032, whereas Bcl-2 protein was not affected. The initial decrease of Mcl-1 protein was the result of transcriptional inhibition rather than cleavage by caspase. Compared with flavopiridol and roscovitine, SNS-032 was more potent, both in inhibition of RNA synthesis and at induction of apoptosis. SNS-032 activity was readily reversible; removal of SNS-032 reactivated RNA polymerase II, which led to resynthesis of Mcl-1 and cell survival. Thus, these data support the clinical development of SNS-032 in diseases that require short-lived oncoproteins for survival.[1] SNS-032 (BMS-387032) is a selective cyclin-dependent kinase (CDK) inhibitor. In this study, we evaluated its effects on primary acute myeloid leukemia (AML) samples (n=87). In vitro exposure to SNS-032 for 48 h resulted in a mean LD(50) of 139±203 nM; Cytarabine (Ara-C) was more than 35 times less potent in the same cohort. SNS-032-induced a dose-dependent increase in annexin V staining and caspase-3 activation. At the molecular level, SNS-032 induced a marked dephosphorylation of serine 2 and 5 of RNA polymerase (RNA Pol) II and inhibited the expression of CDK2 and CDK9 and dephosphorylated CDK7. Furthermore, the combination of SNS-032 and Ara-C showed remarkable synergy that was associated with reduced mRNA levels of the antiapoptotic genes XIAP, BCL2 and MCL1. In conclusion, SNS-032 is effective as a single agent and in combination with Ara-C in primary AML blasts. Treatment with Ara-C alone significantly induced the transcription of the antiapoptotic genes BCL2 and XIAP. In contrast, the combination of SNS-032 and Ara-C suppressed the transcription of BCL2, XIAP and MCL1. Therefore, the combination of SNS-032 and Ara-C may increase the sensitivity of AML cells to the cytotoxic effects of Ara-C by inhibiting the transcription of antiapoptotic genes.[5] |
| 分子式 |
C17H24N4O2S2
|
|---|---|
| 分子量 |
380.53
|
| 精确质量 |
380.134
|
| 元素分析 |
C, 53.66; H, 6.36; N, 14.72; O, 8.41; S, 16.85
|
| CAS号 |
345627-80-7
|
| 相关CAS号 |
345627-80-7;345627-90-9 (HCl);
|
| PubChem CID |
3025986
|
| 外观&性状 |
white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 折射率 |
1.607
|
| LogP |
2.79
|
| tPSA |
133.59
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
454
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S1C(=C([H])N=C1N([H])C(C1([H])C([H])([H])C([H])([H])N([H])C([H])([H])C1([H])[H])=O)SC([H])([H])C1=NC([H])=C(C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])O1
|
| InChi Key |
OUSFTKFNBAZUKL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H24N4O2S2/c1-17(2,3)12-8-19-13(23-12)10-24-14-9-20-16(25-14)21-15(22)11-4-6-18-7-5-11/h8-9,11,18H,4-7,10H2,1-3H3,(H,20,21,22)
|
| 化学名 |
N-[5-[(5-tert-butyl-1,3-oxazol-2-yl)methylsulfanyl]-1,3-thiazol-2-yl]piperidine-4-carboxamide
|
| 别名 |
BMS-387032; SNS 032; BMS387032; SNS032; N-(5-(((5-(tert-Butyl)oxazol-2-yl)methyl)thio)thiazol-2-yl)piperidine-4-carboxamide; N-[5-[(5-tert-butyl-1,3-oxazol-2-yl)methylsulfanyl]-1,3-thiazol-2-yl]piperidine-4-carboxamide; SNS-032 (BMS-387032); BMS 387032; SNS-032
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.47 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.47 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.47 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% PEG400+0.5% Tween80+5% Propylene glycol : 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6279 mL | 13.1396 mL | 26.2791 mL | |
| 5 mM | 0.5256 mL | 2.6279 mL | 5.2558 mL | |
| 10 mM | 0.2628 mL | 1.3140 mL | 2.6279 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00446342 | Completed | Drug: SNS-032 Injection | Multiple Myeloma Mantle Cell Lymphoma |
Sunesis Pharmaceuticals | February 2007 | Phase 1 |
| NCT00292864 | Completed | Drug: SNS-032 Injection | Tumors | Sunesis Pharmaceuticals | January 2006 | Phase 1 |
|
|---|
|