| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 10g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Male F344 rats (five per group, 6-week-old) were given 5% Sodium Erythorbate in feed for 22 weeks. The rats eliminated totals of 203.3 +/- 33.2 mg/100 mL erythorbic acid and 9.0 +/- 5.1 mg/100 mL dehydroerythorbic acid during the study. Ascorbic acid and dehydroascorbic acid were not detected. Urine pH was 6.98 +/-0.31, which was significantly different from that of rats given basal diet alone (6.31 +/- 0.18; p < 0.05). Urine osmolarity also differed significantly from controls; osmolarity was 1378 +/- 277 mOsmol/kg H20 in rats given Sodium Erythorbate and 1756+/- 200 mOsmol/kg H20 in rats of the control group. Crystals were detected in urine of rats given basal diet and Sodium Erythorbate or basal diet alone. Metabolism / Metabolites Male F344 rats (five per group, 6-week-old) were given 5% Sodium Erythorbate in feed for 22 weeks. The rats eliminated totals of 203.3 +/- 33.2 mg/100 mL erythorbic acid and 9.0 +/- 5.1 mg/100 mL dehydroerythorbic acid during the study. Ascorbic acid and dehydroascorbic acid were not detected. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Sodium erythorbate forms white, free-flowing crystals. It is a synthetic antioxidant used in food and cosmetic formulations. Foliar application of sodium erythorbate sprays and dusts are used to control young tree decline in citrus trees and to reduce ozone damage to Thompson seedless grapes. It is also used in hydraulic fracturing mixtures to prevent precipitation of metal oxides (iron control). HUMAN EXPOSURE AND TOXICITY: Sodium erythorbate did not cause chromosomal aberrations or sister chromatid exchanges in cultured human embryo fibroblasts. ANIMAL STUDIES: Sodium erythorbate powder did not cause signs of dermal irritation when applied to the intact and abraded skin of rabbits. Instillation of sodium erythorbate powder to the conjunctival sac of rabbits caused slight and transient reddening of the conjunctiva that cleared within 24 hours. Sodium erythorbate did not cause maternal or fetal toxicity when administered to female rats and mice during gestation by oral intubation at dosages up to 1030 mg/kg/day. Developmental toxicity did not occur after pregnant rats were given up to 5% sodium erythorbate in feed during a 13-week teratogenesis study. It produced negative results in the Ames test, the host-mediated assay using S. typhimurium, chromosomal aberration tests using Chinese hamster ovary fibroblasts, the dominant lethal test using rats, and the B. subtilis rec assay. Sodium erythorbate did cause chromosomal aberrations in rat bone marrow cells in vivo. It did not increase the mitotic recombination frequency of S. cerevisiae D3 in vitro, and did not induce heritable translocation heterozygosity in male mice. Rats given 5% sodium erythorbate in feed for 168 days had no morphological alterations such as hyperplasias of the urinary bladder mucosa. It did not enhance the development of rare spontaneous tumors or transform benign tumors to carcinomas after administration to rats in feed at concentrations up to 2.5%. During a 24-week study, rats given 5% sodium erythorbate in feed had simple hyperplasia of the urinary bladder epithelium. The addition of 1.25-2.5% sodium erythorbate to drinking water did not significantly increase tumor incidence, time to death with tumors, or the distribution of tumors in mice after 96 weeks of treatment. It did not have modifying effects on second-stage carcinogenesis of the nonglandular and glandular stomach, colon, liver, kidneys, mammary gland, ear duct, or thyroid gland, but increased the incidence and average number of lesions of the urinary bladder after initiation with N-butyl-(4-hydroxybutyl)nitrosamine. After administration of sodium erythorbate to rats, they eliminated it in urine as erythorbic acid and dehydroerythorbic acid, whereas ascorbic acid and dehydroascorbic acid were not detected. ECOTOXICITY STUDIES: The acute toxicity of the sodium erythorbate to the freshwater fish rainbow trout (Oncorhynchus myldss) has been investigated and gave a 96-Hour LC50 of greater than 100 mg/L (semi-static). Interactions Effects of 17 environmental chemicals on urinary bladder carcinogenesis were investigated in rats. Male F-344-rats were given orally 0.05 percent N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN). Rats were fed diets containing 5 percent sodium-saccharin, 2 percent sodium-o-phenylphenate (SOPP), 2 percent butylated-hydroxyanisole (BHA), 5 percent sodium-L-ascorbate (SA), 5 percent ascorbic-acid, 5 percent ascorbic-stearate, 5 percent sodium-erythorbate (SE), 0.8 percent ethoxyquin, 0.02 percent N-nitrosopyrrolidine, 0.2 percent methylhydroquinone, 0.2 percent hydroquinone, 0.2 percent resorcinol, 0.8 percent catechol, 0.5 percent pyrogallol, 0.6 percent carbazole, 0.1 percent quinoline, or 1 percent uric-acid. The left ureter was ligated on day 22. Animals were killed after week 24 and autopsied. Urinary bladder, both kidneys, ureter, and liver were stained for light microscopy examination. No cancer was induced in any rat. Papillary or nodular (PN) hyperplasia was induced by BBN in 7 percent of controls. PN hyperplasia incidences and quantitative values were significantly higher in BBN treated rats fed sodium-saccharin, SOPP, BHA, SA, SE, ethoxyquin, and carbazole. Significant differences in incidences and quantitative values of papillomas were observed in BBN treated rats fed sodium-saccharin, SOPP, BHA, SA, and N-nitrosopyrrolidine. Histopathological changes were found in the left kidney, and dilation in the left pelvic space and ureter were common. No changes were found in the right kidney, ureter, or liver. The authors conclude that the ureter ligation system seems to be suitable as a short term method for the screening of bladder carcinogens and promoters. Studies were made on the carcinogenic activity of butylated hydroxyanisole (BHA) in rats, mice, and hamsters and the effect of the antioxidants BHA, butylated hydroxytoluene (BHT), ethoxyquin (EQ), sodium L-ascorbate (SA), ascorbic acid (AA), sodium erythorbate (SE), propyl gallate (PG), and alpha-tocopherol, on two-stage chemical carcinogenesis in rats initiated with N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), 1,2-dimethylhydrazine (DMH), diethylnitrosamine (DEN), 7,12-dimethylbenz(a)anthracene (DMBA), N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN), N-ethyl-N-hydroxyethylnitrosamine (EHEN), or N-methylnitrosourea (MNU). BHA clearly induced squamous cell carcinomas in both the rat and hamster forestomach. The tumorigenic action of crude BHA on the forestomach is largely due to 3-tert-BHA. In two-stage chemical carcinogenesis, BHA promoted MNNG or MNU-initiated forestomach and BBN- or MNU-initiated urinary bladder carcinogenesis and inhibited DEN- or EHEN-initiated liver and DMBA-initiated mammary carcinogenesis. BHT demonstrated promotion potential for urinary bladder and MNU-initiated thyroid carcinogenesis and inhibited DMBA-initiated ear duct carcinogenesis. EQ promoted EHEN-initiated kidney carcinogenesis and inhibited DMBA-initiated mammary and EHEN-initiated liver carcinogenesis. SA promoted forestomach and urinary bladder carcinogenesis and SE likewise enhanced urinary bladder carcinogenesis. alpha-Tocopherol inhibited ear duct carcinogenesis. No effects of any of the antioxidants on glandular stomach carcinogenesis were found. The results clearly demonstrated that antioxidants have different effects (promoting or inhibitory influences) depending on the organ studied and suggest the importance of a whole body approach to their investigation. Studies were conducted on the carcinogenic activity of butylated hydroxyanisole (BHA) in rats and hamsters. To obtain information concerning the mechanism of action of BHA on the forestomach, the following areas were examined: the effects of 12 phenolic compounds structurally related to BHA on the hamster forestomach, the effects of combinations of BHA and other antioxidants on the rat forestomach, and the metabolism of BHA in the forestomach. Also examined were the effects of several antioxidants on two-stage carcinogenesis in rats. Squamous-cell carcinomas were induced in the forestomach of rats and hamsters fed BHA. In a limited study, 1 of 13 hamsters developed a squamous-cell carcinoma. The tumorigenic action of crude BHA on the forestomach was largely due to the action of 3-tert-BHA. p-tert-Butylphenol and 2-tert-butyl-4-methylphenol induced pronounced hyperplasia and papillomas in the hamster forestomach. BHA and other antioxidants, particularly propyl gallate and ethoxyquin, showed additive effects in inducing forestomach hyperplasia and cytotoxicity. Neither BHA nor its metabolites were found in the forestomach epithelium, although small amounts of metabolites were detected in the stomach contents. Thus, a direct action on the stomach epithelium may be exerted by BHA itself or by metabolites formed on interaction of BHA with gastric juice. BHA enhanced forestomach carcinogenesis initiated in rats by N-methyl-N'-nitro-N-nitrosoguanidine or N-methylnitrosourea (MNU) and enhanced urinary bladder carcinogenesis initiated by MNU or N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN). In contrast, it inhibited carcinogenesis initiated in the liver by either diethylnitrosamine or N-ethyl-N-hydroxyethylnitrosamine (EHEN) and mammary carcinogenesis initiated by 7,12-dimethylbenz[a]anthracene (DMBA). BHT promoted urinary bladder carcinogenesis initiated by BBN or MNU and thyroid carcinogenesis initiated by MNU, but inhibited ear-duct carcinogenesis initiated by DMBA. Ethoxyquin promoted EHEN-initiated kidney carcinogenesis, but inhibited both DMBA-initiated mammary and EHEN-initiated liver carcinogenesis. Sodium ascorbate promoted forestomach and urinary bladder carcinogenesis, and sodium erythorbate also enhanced urinary bladder carcinogenesis. Alpha-tocopherol inhibited ear-duct carcinogenesis. No antioxidants tested had any effect on glandular stomach carcinogenesis. Thus antioxidants have independent modifying (promoting or inhibitory) effects in different organs. The modifying effects of antioxidants were examined in a carcinogenesis system after N,N-dibutylnitrosamine treatment. Male F344 rats were given 0.05% N,N-dibutylnitrosamine in their drinking water for 4 wk and then treated with basal diet containing 2% butylated hydroxyanisole (BHA), 1% butylated hydroxytoluene (BHT) with 7 ppm vitamin K, 0.8% ethoxyquin, 5% sodium L-ascorbate, 5% sodium erythorbate, or no added chemical for 32 wk. BHA enhanced forestomach carcinogenesis but did not enhance esophageal carcinogenesis. BHT enhanced esophageal carcinogenesis but did not enhance forestomach carcinogenesis. Ethoxyquin significantly enhanced esophageal tumorigenesis. Neither esophageal nor forestomach carcinogenesis was affected by the other antioxidants evaluated. BHA significantly increased DNA synthesis of the forestomach epithelium, whereas BHT tended to increase that of the esophageal epithelium. Thus, BHA and BHT showed different modifying responses in carcinogenesis of the esophagus and forestomach. For more Interactions (Complete) data for SODIUM ERYTHORBATE (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rats oral > 5 g/kg |
| 其他信息 |
Almost odorless fluffy, white to off-white crystalline powder. Used as an antioxidant and preservative.
See also: Sodium Erythorbate (annotation moved to). |
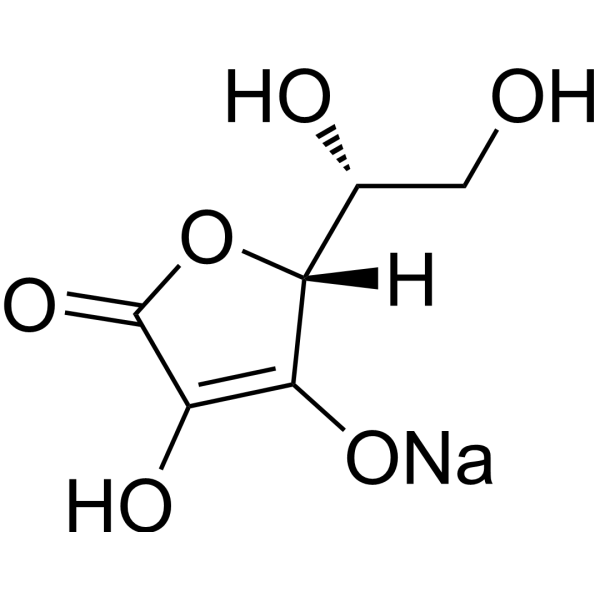
| 分子式 |
C6H7NAO6
|
|---|---|
| 分子量 |
198.1060
|
| 精确质量 |
198.014
|
| CAS号 |
6381-77-7
|
| 相关CAS号 |
Erythorbic acid;89-65-6
|
| PubChem CID |
54680695
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.954g/cm3
|
| 沸点 |
552.7ºC at 760mmHg
|
| 熔点 |
168 - 170ºC
|
| 闪点 |
238.2ºC
|
| tPSA |
110.05
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
237
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C([C@H]([C@@H]1C(=C(C(=O)O1)O)O)O)[O-].[Na+]
|
| InChi Key |
RBWSWDPRDBEWCR-RKJRWTFHSA-N
|
| InChi Code |
InChI=1S/C6H7O6.Na/c7-1-2(8)5-3(9)4(10)6(11)12-5;/h2,5,8-10H,1H2;/q-1;+1/t2-,5-;/m1./s1
|
| 化学名 |
sodium;(2R)-2-[(2R)-3,4-dihydroxy-5-oxo-2H-furan-2-yl]-2-hydroxyethanolate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~252.39 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (12.62 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (12.62 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0477 mL | 25.2385 mL | 50.4770 mL | |
| 5 mM | 1.0095 mL | 5.0477 mL | 10.0954 mL | |
| 10 mM | 0.5048 mL | 2.5239 mL | 5.0477 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。