| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |

| 体外研究 (In Vitro) |
体外活性:螺内酯是强 AR 拮抗剂 (IC50 ~ 77 nM)、弱 GR 拮抗剂 (IC50 ~ 2.4 μM) 和弱 PR 激动剂 (EC50 ~ 740 nM)。螺内酯抑制大鼠前列腺细胞核中雄甾烷酮的结合以及大鼠前列腺细胞质中雄甾烷酮的特异性结合。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
螺内酯(1 毫克/天)对大鼠具有抗雄激素活性。螺内酯(1 mg/大鼠)的单次预处理可抑制[3H]睾酮示踪剂剂量诱导的前列腺特异性且可饱和的激素摄取。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The mean time to reach peak plasma concentration of spironolactone and the active metabolite, canrenone, in healthy volunteers is 2.6 and 4.3 hours, respectively. Food increased the bioavailability of spironolactone (as measured by AUC) by approximately 95.4%. The metabolites are excreted primarily in the urine and secondarily in bile. Metabolites of spironolactone are excreted in urine (42-56%) and in the feces (14.2-14.6%). No unmetabolized spironolactone is present in the urine. At the time spironolactone was introduced for clinical use, its bioavailability was inadequate, and this was improved by preparing the drug in finely powdered or micronized form. The absolute bioavailability was indirectly estimated at approximately 73%, which was enhanced in the presence of food. Nearly all absorbed spironolactone (> 90 %) is bound to plasma proteins and, with repeated dosing, a steady state is achieved within 8 days. After oral intake of a 100-mg dose, the plasma half-time of spironolactone was 1-2 h, the time to maximum plasma concentration was 2-3.2 hr, the maximum blood concentration was 92-148 ng/mL, the area under the concentration--time (0-24 hr) curve was 1430-1541 ng/mL per hr and the elimination half-time was 18-20 hr. The disposition of (14)C spironolactone was studied in male rats, female dogs and female monkeys after intravenous or oral administration of 5 mg/kg bw. Gastrointestinal absorption was estimated to be 82% in rats, 62% in dogs and 103% in monkeys. Spironolactone was extensively metabolized in all three species, and the metabolites were excreted primarily in the urine and feces. The amount of radiolabel excreted in urine or feces of all three species was similar after intravenous and after oral dosing. In monkeys, as in humans, the amounts excreted in urine and feces were about equal, while fecal excretion predominated in rats and dogs as a result of biliary excretion. After the oral dose, the percentage of urinary excretion was 4.7% in rats, 18% in dogs and 46% in monkeys. The high excretion of radiolabel in the feces of rats (90%) after intravenous administration shows the importance of biliary excretion for that species. Species differences were also noted in the biotransformation of spironolactone. ... Absorption of spironolactone from the GI tract depends on the formulation in which it is administered. Currently available formulations of spironolactone are well absorbed from the GI tract and bioavailability of the drug exceeds 90% when compared to an optimally absorbed spironolactone solution in polyethylene glycol 400. Following a single oral dose of spironolactone, peak serum concentrations of the drug occur within 1-2 hours, and peak serum concentrations of its principal metabolites are attained within 2-4 hours. When spironolactone is administered concomitantly with food, peak serum concentrations and areas under the serum concentration-time curves (AUCs) of the drug and, to a lesser degree, its principal metabolites are increased substantially compared with the fasting state... Spironolactone and canrenone, a major metabolite of the drug, are both more than 90% bound to plasma proteins. Spironolactone or its metabolites may cross the placenta. Canrenone, a major metabolite of spironolactone, is distributed into milk. Metabolism / Metabolites Spironolactone is rapidly and extensively metabolized to form different metabolites. A group of metabolites are formed when sulfur of spironolactone is removed, such as [canrenone]. Sulfur is retained in another group of metabolites, including 7-alpha (α)-thiomethylspironolactone (TMS) and 6-beta (ß)-hydroxy-7-alpha (α)-thiomethylspirolactone (HTMS). Spironolactone is firstly deacetylated to 7-α-thiospironolactone. 7-α-thiospironolactone is S-methylated to TMS, which is the primary metabolite, or dethioacetylated to canrenone. TMS and HTMS can be further metabolized. In humans, the potencies of TMS and 7-α-thiospirolactone in reversing the effects of the synthetic mineralocorticoid, fludrocortisone, on urinary electrolyte composition were approximately a third relative to spironolactone. However, since the serum concentrations of these steroids were not determined, their incomplete absorption and/or first-pass metabolism could not be ruled out as a reason for their reduced _in vivo_ activities. Spironolactone is rapidly and extensively metabolized to compounds that are excreted in the urine and faeces. It undergoes enterohepatic recirculation, but no unchanged drug appears in urine or feces. The metabolites of spironolactone can be divided into two main groups: those in which the sulfur moiety is retained and those in which the sulfur is removed by dethioacetylation. For many years, it was thought that the dethioacetylated metabolite, canrenone, was the major metabolite; however, with more specific analytical methods such as HPLC, 7alpha-thiomethylspirolactone was recognized as the major metabolite of spironolactone. This metabolite is formed by hydrolysis of the thioacetate group to form 7alpha-thiospirolactone (as an intermediate), followed by S-methylation to 7alpha-thiomethylspirolactone. This can then be hydroxylated to form 6â-hydroxy-7alpha-thiomethylspirolactone and oxidized to form 7alpha-methylsulfinyl- and 7alpha-methylsulfonylspirolactone or via sulfoxidation to form 6alpha-hydroxy-7alpha-methylsulfinyl- and 6beta-hydroxy-7alpha-methylsulfonylspirolactone. For formation of the group of metabolites in which sulfur is removed, 7alpha-thiomethylspirolactone is also dethioacetylated to canrenone, which is further metabolized by three pathways: hydrolysis of the gamma-lactone ring to form canrenoate, which is excreted in the urine as a glucuronic ester, and, next, hydroxylation to form 15alpha-hydroxy-canrenone or reduction to produce several di-, tetra- and hexa-hydro derivatives. Canrenone and canrenoate are in equilibrium with one another. Not only spironolactone but several of its metabolites have biological activity; in decreasing order of potency, these are 7alpha-thiospirolactone, 7alpha-thiomethylspirolactone and canrenone. Species differences were ... noted in the biotransformation of spironolactone. Canrenone was a principal extractable metabolite in rat and dog plasma, whereas in monkeys and humans, both canrenone and a very polar, unidentified metabolite were the major constituents. In the urine of all four species, canrenone was a principal constituent. Notable species differences in the metabolites of spironolactone in the feces were found, the pattern of metabolites in dog feces being markedly different from that in rats, monkeys or humans. Overall, it was concluded that the disposition and metabolism of spironolactone in monkeys, rather than that in rats or dogs, is closest to that in humans. SIX METABOLITES OF SPIRONOLACTONE ... /HAVE/ BEEN DETECTED IN URINE OF TREATED SUBJECTS. ... /ONE IS/ DETHIOACETYLATED COMPD CANRENONE, 3-(3-OXO-17BETA-HYDROXY-4,6-ANDRO-STADIEN-17ALPHA-YL)PROPIONIC ACID GAMMA-LACTONE... A FLUOROMETRIC METHOD WAS USED TO DETERMINE CONCN OF CANRENONE IN MILK FOLLOWING INGESTION OF SPIRONOLACTONE (ALDACTONE) 25 MG TWICE DAILY IN 28-YR-OLD FEMALE. Rapidly and extensively metabolized. The metabolic pathway of spironolactone is complex and can be divided into two main routes: those in which the sulfur moiety is retained and those in which the sulfur moiety is removed by dethioacetylation. Spironolactone is transformed to a reactive metabolite that can inactivate adrenal and testicular cytochrome P450 enzymes. It also has anti-androgenic activity. Route of Elimination: The metabolites are excreted primarily in the urine and secondarily in bile. Half Life: 10 minutes Biological Half-Life The mean half-life of spironolactone is 1.4 hours. The mean half-life values of its metabolites, including canrenone, TMS, and HTMS are 16.5, 13.8, and 15 hours, respectively. ... Nearly all absorbed spironolactone (> 90 %) is bound to plasma proteins and, with repeated dosing, a steady state is achieved within 8 days. After oral intake of a 100-mg dose, the plasma half-time of spironolactone was 1-2 h, the time to maximum plasma concentration was 2-3.2 hr, the maximum blood concentration was 92-148 ng/mL, the area under the concentration--time (0-24 hr) curve was 1430-1541 ng/mL per hr and the elimination half-time was 18-20 hr. Following a single oral dose in healthy adults, the half-life of spironolactone averages 1.3-2 hours, and the half-life of 7alpha-thiomethylspironolactone averages 2.8 hours. The half-life of canrenone reportedly ranges from 13-24 hours. In multiple-dose studies, the steady-state plasma elimination half-life of canrenone averaged 19.2 hours when 200 mg of spironolactone was administered daily as a single dose and averaged 12.5 hours when 200 mg of the drug was administered daily in 4 equally divided doses. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Spironolactone is a specific pharmacologic antagonist of aldosterone, acting primarily through competitive binding of receptors at the aldosterone-dependent sodium-potassium exchange site in the distal convoluted renal tubule. Spironolactone causes increased amounts of sodium and water to be excreted, while potassium is retained. Spironolactone acts both as a diuretic and as an antihypertensive drug by this mechanism. It may be given alone or with other diuretic agents which act more proximally in the renal tubule. Aldosterone interacts with a cytoplasmic mineralocorticoid receptor to enhance the expression of the Na+, K+-ATPase and the Na+ channel involved in a Na+ K+ transport in the distal tubule . Spironolactone bind to this mineralcorticoid receptor, blocking the actions of aldosterone on gene expression. Aldosterone is a hormone; its primary function is to retain sodium and excrete potassium in the kidneys. Hepatotoxicity Clinically apparent liver injury from spironolactone is rare and only a few instances have been reported as isolated case reports. The liver injury typically arises after 4 to 8 weeks of therapy and the pattern of serum enzyme elevations is usually hepatocellular or mixed. Immunoallergic features (rash, fever, eosinophilia) are rare as is autoantibody formation. Recovery has occurred within 1 to 3 months of stopping and all cases have been mild and self-limited in course (Case 1). Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited data indicate that spironolactone is poorly excreted into breastmilk. Case of mothers breastfeeding during spironolactone therapy reported no adverse effects in infants. Spironolactone appears to be acceptable to use during breastfeeding. ◉ Effects in Breastfed Infants In 17-day-old breastfed (extent not stated) infant whose mother was taking 25 mg of spironolactone 4 times daily since pregnancy, serum sodium and potassium remained normal.[1] Spironolactone 75 mg every other day was taken orally by a mother while nursing a newborn. She was also taking 400 mg of bretylium tosylate every 8 hours, atenolol 25 mg daily, propranolol 20 mg 3 times a day, and multivitamin, potassium and magnesium supplements. Jaundice, thought to be unrelated to the drug, occurred at 60 hours of age, but resolved. The infant had appropriate weight gain and development during the first 4 months of life.[2] A transgender woman took and spironolactone 50 mg twice daily to suppress testosterone, domperidone 10 mg three times daily, increasing to 20 mg four times daily, oral micronized progesterone 200 mg daily and oral estradiol to 8 mg daily and pumped her breasts 6 times daily to induce lactation. After 3 months of treatment, estradiol regimen was changed to a 0.025 mg daily patch and the progesterone dose was lowered to 100 mg daily. Two weeks later, she began exclusively breastfeeding the newborn of her partner. Breastfeeding was exclusive for 6 weeks, during which the infant's growth, development and bowel habits were normal. The patient continued to partially breastfeed the infant for at least 6 months.[3] A woman with Gitleman syndrome took spironolactone in an unspecified dosage along with potassium and magnesium supplements for at least 4 months while breastfeeding her infant. No adverse infant effects were reported.[4] ◉ Effects on Lactation and Breastmilk Intense diuresis can suppress lactation;[5,6] however, it is unlikely that spironolactone alone is sufficiently potent to cause this effect. Spironolactone can cause gynecomastia. The estimated risk is 52 cases per 1000 patients treated, which is 8.4 times the baseline risk.[7] A transgender woman was taking sublingual estradiol 4 mg twice daily, spironolactone 100 mg twice daily and progesterone 200 mg at bedtime for gender-affirming therapy. In order to prepare for the birth of the infant being carried by her partner, sublingual estradiol was increased to 6 mg twice daily and progesterone was increased to 400 mg at bedtime. Domperidone 10 mg twice daily was also started to increase serum prolactin levels and later increased to 20 mg 4 times daily. Before the delivery date, progesterone was stopped, spironolactone was decreased to 100 mg daily and estradiol was changed to 25 mcg per day transdermally. At day 59 postpartum, estradiol was changed to 2 mg per day sublingually and spironolactone was increased to 100 mg twice daily. The patient was able to produce up to 240 mL of milk daily containing typical macronutrient and oligosaccharide levels.[8] Protein Binding Spironolactone and its metabolites are more than 90% bound to plasma proteins. Spironolactone and canrenone bind to serum albumin and alpha 1-acid glycoprotein. Toxicity Data The oral LD50 of spironolactone is greater than 1,000 mg/kg in mice, rats, and rabbits. Interactions Aspirin has been shown to slightly reduce the natriuretic effect of spironolactone in healthy individuals, possibly by reducing active renal tubular secretion of canrenone, the active metabolite of spironolactone. However, the hypotensive effect of spironolactone and its effect on urinary potassium excretion in hypertensive patients are apparently not affected. Until more clinical data are available on this potential interaction, patients receiving both drugs should be monitored for signs and symptoms of decreased clinical response to spironolactone. Spironolactone reportedly reduces vascular responsiveness to norepinephrine and regional or general anesthesia should be used with caution in patients receiving spironolactone. In an initial experiment, 70 female Sprague-Dawley rats, approximately 50 days of age (weight, 150-180 g), received a single dose of 40 mg 7,12-dimethylbenz[a]- anthracene (DMBA) dissolved in 2 mL of corn oil by oral gavage. Of these rats, 20 also received spironolactone (pharmaceutical-grade) at a dose of 100 mg/kg bw in 1 mL of distilled water by oral gavage twice daily for 7 days, starting 4 days before DMBA administration. The study was terminated 150 days after DMBA treatment, and the mammary tumour incidence determined by palpation. The incidence of palpable mammary tumours was reduced from 21/24 in the group receiving DMBA alone to 3/14 in that given DMBA plus spironolactone. In a second experiment, 80 female Sprague-Dawley rats received an intravenous injection into the jugular vein of 2 mg of DMBA in 0.4 mL of oil emulsion once daily on days 1, 4 and 7. Two days before the first DMBA injection, 40 of these rats received spironolactone (pharmaceutical-grade) at a dose of 100 mg/kg bw in 1 mL of distilled water by oral gavage twice daily for 12 consecutive days. On termination of the study 147 days after the start of DMBA treatment, mammary tumours were found at necropsy in 32/32 rats receiving DMBA alone and 23/36 rats receiving DMBA plus spironolactone (p < 0.001). Salicylates may reduce the tubular secretion of canrenone and decrease the diuretic efficacy of spironolactone, and spironolactone may alter the clearance of digitalis glycosides. For more Interactions (Complete) data for SPIRONOLACTONE (13 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat ip 277 mg/kg LD50 Mouse ip 260 mg/kg LD50 Rabbit ip 866 mg/kg LD50 Rabbit oral > 1000 mg/kg For more Non-Human Toxicity Values (Complete) data for SPIRONOLACTONE (6 total), please visit the HSDB record page. |
||
| 参考文献 |
J Biol Chem.2010 Sep 24;285(39):29932-40;Mol Cell Endocrinol.1974 Dec;2(1):59-67.
|
||
| 其他信息 |
Therapeutic Uses
Aldosterone Antagonists; Diuretics /EXPL THER:/ Aldosterone is important in the pathophysiology of heart failure. In a doubleblind study, we enrolled 1663 patients who had severe heart failure and a left ventricular ejection fraction of no more than 35 percent and who were being treated with an angiotensin-converting-enzyme inhibitor, a loop diuretic, and in most cases digoxin. A total of 822 patients were randomly assigned to receive 25 mg of spironolactone daily, and 841 to receive placebo. The primary end point was death from all causes. The trial was discontinued early, after a mean follow-up period of 24 months, because an interim analysis determined that spironolactone was efficacious. There were 386 deaths in the placebo group (46 percent) and 284 in the spironolactone group (35 percent; relative risk of death, 0.70; 95 percent confidence interval, 0.60 to 0.82; P<0.001). This 30 percent reduction in the risk of death among patients in the spironolactone group was attributed to a lower risk of both death from progressive heart failure and sudden death from cardiac causes. The frequency of hospitalization for worsening heart failure was 35 percent lower in the spironolactone group than in the placebo group (relative risk of hospitalization, 0.65; 95 percent confidence interval, 0.54 to 0.77; P<0.001). In addition, patients who received spironolactone had a significant improvement in the symptoms of heart failure, as assessed on the basis of the New York Heart Association functional class (P<0.001). Gynecomastia or breast pain was reported in 10 percent of men who were treated with spironolactone, as compared with 1 percent of men in the placebo group (P<0.001). The incidence of serious hyperkalemia was minimal in both groups of patients. MEDICATION (VET): Spironolactone can be used with furosemide to control ascites. MEDICATION (VET): Spironolactone is used most frequently and is a competitive antagonist of aldosterone. Aldosterone is elevated in animals with congestive heart failure in which the renin-angiotensin system is activated in response to hyponatremia, hyperkalemia, and reductions in blood pressure or cardiac output. Aldosterone is responsible for increasing sodium and chloride reabsorption and potassium and calcium excretion from renal tubules. Spironolactone competes with aldosterone at its receptor site, causing a mild diuresis and potassium retention. For more Therapeutic Uses (Complete) data for SPIRONOLACTONE (12 total), please visit the HSDB record page. Drug Warnings Spironolactone is an aldosterone antagonist that acts on the mineralocorticoid receptor. It is a potassium-sparing diuretic, and hyperkalemia is the most common and potentially serious complication of therapy. Impaired kidney function appears to increase this risk, as does supplementation with potassium chloride. Excessive diuresis can also lead to dehydration and hyponatraemia. A number of endocrine effects have also been reported, the most common of which is gynaecomastia, with a dose-related incidence of 7-52%. This side-effect is reversible and disappears upon discontinuation of therapy. Other endocrine effects include loss of sexual potency in men and menstrual irregularity, amenorrhea, breast engorgement and chloasma in women. These effects are probably due to interaction of spironolactone with the androgen receptor. There are a few isolated case reports of idiosyncratic drug reactions, including one case of hepatitis and several cases of agranulocytosis. Approximately 10 cases have been reported of allergic contact dermatitis after topical application of spironolactone for various dermal indications involving its antiandrogen activity. Maternal Medication usually Compatible with Breast-Feeding: Spironolactone: Reported Sign or Symptom in Infant or Effect on Lactation: None. /From Table 6/ POTENTIAL ADVERSE EFFECTS ON FETUS: May cross placenta. No controlled studies performed, but no known teratogenic effects. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: Active metabolite (canrenone) excreted in breast milk. FDA Category: C (C = Studies in laboratory animals have revealed adverse effects on the fetus (teratogenic, embryocidal, etc.) but there are no controlled studies in pregnant women. The benefits from use of the drug in pregnant women may be acceptable despite its potential risks, or there are no laboratory animal studies or adequate studies in pregnant women.) /From table II/ For more Drug Warnings (Complete) data for SPIRONOLACTONE (17 total), please visit the HSDB record page. Pharmacodynamics Spironolactone has a potassium-sparing diuretic effect. It promotes sodium and water excretion and potassium retention. It increases renin and aldosterone levels. Spironolactone is a mineralocorticoid receptor antagonist and has a low affinity for the glucocorticoid receptor. It also exhibits progestogenic and anti-androgenic actions as it binds to the androgen receptor and, to a lesser extent, estrogen and progesterone receptors. Spironolactone exhibits anti-inflammatory effects. |
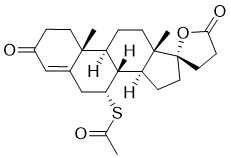
| 分子式 |
C24H32O4S
|
|
|---|---|---|
| 分子量 |
416.57
|
|
| 精确质量 |
416.202
|
|
| CAS号 |
52-01-7
|
|
| 相关CAS号 |
Spironolactone-d7;Spironolactone-d3;Spironolactone-d3-1
|
|
| PubChem CID |
5833
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
597.0±50.0 °C at 760 mmHg
|
|
| 熔点 |
207-208 °C(lit.)
|
|
| 闪点 |
302.3±18.1 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.586
|
|
| LogP |
3.12
|
|
| tPSA |
85.74
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
818
|
|
| 定义原子立体中心数目 |
7
|
|
| SMILES |
CC(=O)S[C@@H]1CC2=CC(=O)CC[C@@]2([C@@H]3[C@@H]1[C@@H]4CC[C@]5([C@]4(CC3)C)CCC(=O)O5)C
|
|
| InChi Key |
LXMSZDCAJNLERA-NMFLDQOASA-N
|
|
| InChi Code |
InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24-/m0/s1
|
|
| 化学名 |
S-((7R,8R,9S,10R,13S,14S,17S)-10,13-dimethyl-3,5-dioxo-1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16-hexadecahydro-3H-spiro[cyclopenta[a]phenanthrene-17,2-furan]-7-yl) ethanethioate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.00 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.00 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.00 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4006 mL | 12.0028 mL | 24.0056 mL | |
| 5 mM | 0.4801 mL | 2.4006 mL | 4.8011 mL | |
| 10 mM | 0.2401 mL | 1.2003 mL | 2.4006 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Spironolactone Safety in African Americans with Mild Cognitive Impairment and Early Dementia
CTID: NCT04522739
Phase: Phase 4 Status: Active, not recruiting
Date: 2024-10-15
|
|---|