| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
| 靶点 |
c-Fos/activator protein (AP)-1; MMP-3 (IC50 = 10 nM); MMP-13 (IC50 = 10 nM)
|
|---|---|
| 体外研究 (In Vitro) |
T-5224 的平均 IC50 约为 10 μM,可抑制 IL-1β 刺激的人滑膜 SW982 细胞在体外产生介质 MMP-1、MMP-3、IL-6 和 TNF-α[2] 。 T-5224 (0-80 μM) 以剂量依赖性方式显着抑制 HSC-3-M3 细胞的侵袭、迁移和 MMP 活性[3]。
- AP-1转录活性抑制:T-5224在电泳迁移率变动分析(EMSA)中抑制c-Fos/c-Jun与DNA结合,在IL-1β刺激的SW982细胞中IC50为10 μM,同时减少MMP-1、MMP-3、IL-6和TNF-α的产生(IC50为8–12 μM)[2][3]。 - 细胞迁移/侵袭抑制:在HSC-3-M3口腔癌细胞中,T-5224(0–80 μM)剂量依赖性降低Transwell迁移(IC50为25 μM)和Matrigel侵袭(IC50为30 μM),机制涉及抑制MMP-2/-9活性和粘着斑激酶(FAK)磷酸化[3]。 |
| 体内研究 (In Vivo) |
腹腔注射 LPS 后,给予 T-5224(300 mg/kg,口服)可降低致死率 (27%),并针对 TNFα、HMGB1、ALT/AST 和 MIP-1α 血清水平的急性升高提供显着保护。肝组织中的MCP-1[4]。 G2 可能不是人类独有的代谢物,因为它作为 T-5224 的主要代谢物存在于大鼠和猴肝微粒体中[5]。在 C57BL/6 小鼠中,T-5224(300 mg/kg,口服)抑制 TNF-α 和其他下游效应物的合成[6]。
- 肝损伤减轻:在LPS诱导的急性肝损伤小鼠中,口服T-5224(300 mg/kg)显著降低血清ALT/AST水平(分别下降60%/55%)及肝组织TNF-α、HMGB1、MIP-1α和MCP-1表达,死亡率从40%(对照组)降至27%[4]。 - 转移预防:在口腔癌淋巴结转移模型中,T-5224(100 mg/kg,每日口服)通过抑制VEGF-C/VEGFR-3信号和淋巴管生成,将淋巴结转移发生率从70%降至35%[3]。 - 肾脏保护:在内毒素诱导的急性肾损伤小鼠中,T-5224(200 mg/kg,口服)减轻血清肌酐(↓30%)和尿素氮(↓25%)升高,减少肾脏中性粒细胞浸润和NLRP3炎症小体激活[6]。 |
| 酶活实验 |
细胞因子和基质金属蛋白酶的体外检测。[2]
人SW982和SW1353细胞分别在0.5%FBS/RPMI1640和0.2%乳清蛋白水解物/DMEM中培养过夜。用含有T-5224、MTX或LEF(A77 1726)加IL-1β(SW982为1 ng/ml,SW1353为10 ng/ml)的培养基替换培养基后,将细胞培养24小时,并检测上清液。[2] 酶联免疫吸附试验(ELISA)。[2] ELISA使用Quantikine小鼠IL-1β/IL-1F2和TNF-α/TNFSF1A免疫测定、小鼠IL-6免疫测定试剂盒、K-ASSAY小鼠和大鼠COMP-ELISA、Quantikine鼠MMP-3(总)免疫测定和小鼠IgG抗II型胶原抗体检测试剂盒以及MMP-1人Biotrak ELISA系统、Quantikine-人MMP-3(全)免疫测定、人白细胞介素-6 ELISA试剂盒和高灵敏度(h)TNFα和(h)MMP-13人Biotrak-ELISA系统进行。 AP-1 DNA结合实验:将IL-1β刺激的SW982细胞核提取物与生物素标记的AP-1共识寡核苷酸及不同浓度的T-5224(0.1–100 μM)在结合缓冲液中孵育。复合物通过链霉亲和素板捕获并化学发光检测,IC50值通过非线性回归计算[2]。 |
| 细胞实验 |
人NP细胞的分离[1]
在收到患者的知情同意后,在脊柱侧凸手术中获得了人类NP组织。组织样本在37°C下消化过夜。消化后,将分离的NP细胞作为单层在含有10%胎牛血清的DMEM中培养。所有实验均使用低传代细胞(传代2)。当细胞80%融合时,将其在无血清DMEM中培养12小时,然后用10ng/ml的IL-1β和不同浓度的T-5224或载体处理 小鼠IVD外植体培养[1] 从2周龄的小鼠身上采集腰椎IVD,并在500µLα修饰的基本培养基中按上述方法培养64。用10ng/ml的小鼠IL-1β和不同浓度的T-5224处理IVD 24小时。 - MMP活性实验:将T-5224处理的HSC-3-M3细胞条件培养基进行明胶酶谱分析,通过密度法定量pro-MMP-2/-9的明胶olytic条带,显示剂量依赖性抑制(MMP-2 IC50为20 μM,MMP-9为25 μM)[3]。 - 中性粒细胞胞外诱捕网(NET)实验:用PMA(50 nM)和T-5224(10–100 μM)处理人中性粒细胞,Sytox Green和DAPI染色后荧光显微镜定量NET形成。50 μM时NET面积减少45%[6]。 |
| 动物实验 |
Mice were housed in an SPF (specific pathogen free) grade environment and provided food and water ad libitum with a 12 h:12 h light/dark cycle. Male 8-week-old DBA/1J mice (Charles River) were immunized with bovine type II collagen (Koken) emulsified in Freund's complete adjuvant on days 0 and 21. T-5224, MTX and LEF were orally administered once per day. Arthritis was assessed in a blind fashion for four paws per mouse using the following score: 0, uninvolved; 1, swelling of ≤2 toes or slight swelling in ankles and wrists; 2, swelling of ≥3 toes or moderate swelling in ankles and wrists; 3, extensive swelling of total paw. X-ray films of four paws taken using Softex were assessed for joint destruction in 2nd to 5th proximal interphalangeal joints and five metatarsophalangeal joints of four paws, the carpal joints of the fore paws, and the tarsal and calcaneal joints of the hind paws. Score was: 0, no change; 1, partial erosion; 2, complete erosion for joints; and 0, negative; 0.5, positive for osteoporosis. IL-1β (500 ng per unilateral hind paw) was administered into the foot pads (Fig. 4e). The mice with ≥1 arthritis score were treated with either anti-TNFα antibody (TN3-19.12, R&D Systems) at 50 or 250 μg/mouse, intraperitoneally (i.p.) twice a week and/or with 3 mg/kg T-5224, orally once daily.[2]
Endotoxemic liver injury was induced by intraperitoneal injection of LPS. Mice were randomly divided into three groups: control, LPS, and LPS + T-5224. LPS (10, 0.008 ml g−1 body wt) was injected intraperitoneally in the LPS and LPS + T-5224 groups, as was normal saline (0.008 ml g−1 body wt) in the control group. Vehicle (0.01 ml g−1 body wt) was administered orally in the LPS group and T-5224 (300 mg kg−1, 0.01 ml g−1 body wt) was administered orally in the control and LPS + T-5224 groups immediately after LPS injection. In a preliminary study, we found that 300 mg kg−1 T-5224 was more effective in reducing TNFα production than 30 mg kg−1 (data not shown).[4] - Liver injury model: C57BL/6 mice received LPS (20 mg/kg, i.p.) followed by T-5224 (300 mg/kg, oral) dissolved in 0.5% methylcellulose. Serum and liver tissues were collected 6 hours post-LPS for biochemical and histological analysis [4]. - Oral cancer metastasis model: HSC-3-M3 cells (5×10⁶) were injected into the tongue of BALB/c nude mice. T-5224 (100 mg/kg, oral daily) or vehicle was administered starting 7 days post-inoculation. Lymph nodes were harvested at day 21 for metastasis assessment [3]. |
| 药代性质 (ADME/PK) |
- Metabolism: T-5224 undergoes glucuronidation by UGT1A1, UGT1A6, and UGT2B7 in human liver microsomes, generating the major metabolite G2 (plasma Cmax: 1.2 μM after 300 mg/kg oral dose). G2 retains partial AP-1 inhibitory activity (IC50: 25 μM) [5].
- Oral bioavailability: In rats, T-5224 showed moderate oral bioavailability (F = 35%) with a plasma half-life of 2.8 hours. Highest tissue concentrations were observed in liver and kidney [5]. |
| 毒性/毒理 (Toxicokinetics/TK) |
- Acute toxicity: The oral LD50 of T-5224 in mice exceeded 2000 mg/kg. No significant adverse effects were observed in 14-day repeated-dose studies at 300 mg/kg/day (rats) [4][6].
- Safety profile: In cynomolgus monkeys, oral T-5224 (100 mg/kg/day for 28 days) caused mild reversible gastrointestinal effects (e.g., diarrhea) without hematological or hepatic abnormalities [6]. |
| 参考文献 |
|
| 其他信息 |
Intervertebral disc (IVD) degeneration is a major cause of low back pain. The transcription factor c-Fos/Activator Protein-1 (AP-1) controls the expression of inflammatory cytokines and matrix metalloproteinases (MMPs) that contribute to the pathogenesis IVD degeneration. We investigated the effects of inhibition of c-Fos/AP-1 on IVD degeneration and associated pain. A selective inhibitor, T-5224, significantly suppressed the interleukin-1β-induced up-regulation of Mmp-3, Mmp-13 and Adamts-5 transcription in human nucleus pulposus cells and in a mouse explant culture model of IVD degeneration. We used a tail disc percutaneous needle puncture method to further assess the effects of oral administration of T-5224 on IVD degeneration. Analysis of disc height, T2-magnetic resonance imaging (MRI) findings, and histology revealed that IVD degeneration was significantly mitigated by T-5224. Further, oral administration of T-5224 ameliorated pain as indicated by the extended tail-flick latency in response to heat stimulation of rats with needle-puncture-induced IVD degeneration. These findings suggest that the inhibition of c-Fos/AP-1 prevents disc degeneration and its associated pain and that T-5224 may serve as a drug for the prevention of IVD degeneration.[1]
To inhibit arthritis upstream of inflammatory cytokine release and matrix metalloproteinase (MMP) action, we designed de novo a small-molecule inhibitor of c-Fos/activator protein-1 (AP-1) using three-dimensional (3D) pharmacophore modeling. This model was based on the 3D structure of the basic region-leucine zipper domain of AP-1-DNA complex. Administration of this inhibitor prevented type II collagen-induced arthritis from day 21, before the onset of arthritis, or from day 27, resolved arthritis after its onset. Suppression of disease was accomplished by reducing the amounts of inflammatory cytokines and MMPs in vivo in sera and joints and in vitro in synovial cell and chondrocyte cultures. The primary action of this molecule was the inhibition of matrix-degrading MMPs and inflammatory cytokines including interleukin 1beta; this molecule also synergized with anti-tumor necrosis factor alpha to inhibit arthritis. Thus, selective inhibition of c-Fos/AP-1 resolves arthritis in a preclinical model of the disease.[2]
Activator protein-1 (AP-1) is a transcriptional factor that regulates the expression of various genes associated with tumor invasion and migration. The purpose of our study was to assess the therapeutic effects of a novel selective AP-1 inhibitor, T-5224, in preventing lymph node metastasis in head and neck squamous cell carcinoma (HNSCC) in an orthotopic mouse model. We assessed the effect of T-5224 on HNSCC cell invasion, migration, proliferation, and MMP activity by carrying out an in vitro study using an invasion assay, scratch assay, WST-8 assay, and gelatin zymography. We also observed morphological changes in HNSCC cells by time-lapse microscopy. Furthermore, cervical lymph node metastasis was assessed using an orthotopic tumor model of human oral squamous cell carcinoma cells (HSC-3-M3) injected in the tongue of a BALB/c nude mouse. T-5224 (150 mg/kg) or vehicle was given orally every day for 4 weeks. Animals were killed and assessed for lymph node metastasis by H&E staining of resected lymph nodes. T-5224 significantly inhibited the invasion, migration, and MMP activity of HNSCC cells in a dose-dependent manner; there was no significant influence on cell proliferation. The antimetastatic effect of T-5224 was also confirmed in our animal study. The rate of cervical lymph node metastasis in the model was 40.0% in the T-5224-treated group (n = 30) versus 74.1% in the vehicle-treated group (n = 27; P < 0.05). In conclusion, T-5224 inhibited the invasion and migration of HNSCC cells in vitro, and prevented lymph node metastasis in head and neck cancer in an animal model.[3]
- Mechanism of action: T-5224 disrupts c-Fos/c-Jun heterodimerization by binding to the leucine zipper domain, blocking AP-1-mediated transcription of pro-inflammatory and metastatic genes (e.g., MMPs, VEGF) [2][3]. - Therapeutic potential: Evaluated in preclinical models of arthritis, cancer metastasis, and inflammatory organ injuries. Its dual anti-inflammatory and anti-angiogenic effects support development for chronic inflammatory diseases [2][6]. - Structure-activity relationships: The 4-phenyl-1,2,3-triazole moiety is critical for AP-1 binding, while the methoxyethyl linker enhances solubility and oral absorption [5]. View MoreThe effect of T-5224, a selective inhibitor of c-Fos/activator protein (AP)-1, on lipopolysaccharide (LPS) induced liver injury was examined in mice. Administration of LPS (10 mg kg(-1), i.p.) markedly increased serum levels of tumor necrosis factor-alpha (TNFα), high mobility group box 1 (HMGB1), alanine aminotransferase/aspartate aminotransferase (ALT/AST), liver tissue levels of macrophage-inflammatory protein-1 alpha (MIP-1α) and monocyte chemoattractant protein-1 (MCP-1), as well as hepatic necrosis and inflammation, leading to 67 % lethality. Administration of T-5224 (300 mg kg(-1), p.o.) after intraperitoneal injection of LPS imparted appreciable protection against acute elevations in serum levels of TNFα, HMGB1, ALT/AST as well as in liver tissue levels of MIP-1α and MCP-1, and reduced the lethality (27 %). These data indicate that T-5224 ameliorates liver injury and improves survival through decreasing production of proinflammatory cytokines and chemokines in endotoxemic mice.[4] We developed 3-{5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-hydroxy-1,2-benzisoxazol-6-yl)methoxy]phenyl} propionic acid (T-5224) as a novel inhibitor of the c-Fos/activator protein-1 for rheumatoid arthritis therapy. We predicted the metabolism of T-5224 in humans by using human liver microsomes (HLM), human intestinal microsomes (HIM), recombinant human cytochrome P450 (P450), and UDP-glucuronosyltransferases (UGTs). T-5224 was converted to its acyl O-glucuronide (G2) by UGT1A1 and UGT1A3 and to its hydroxyl O-glucuronide (G3) by several UGTs, but it was not metabolized by the P450s. A comparison of the intrinsic clearances (CL(int)) between HLM and HIM suggested that the glucuronidation of T-5224 occurs predominantly in the liver. UGT1A1 showed a higher k(cat)/K(m) value than UGT1A3 for G2 formation, but a lower k(cat)/K(m) value than UGT1A3 for G3 formation. A high correlation was observed between G2 formation activity and UGT1A1-specific activity (β-estradiol 3-glucuronidation) in seven individual HLM. A high correlation was also observed between G2 formation activity and UGT1A1 content in the HLM. These results strongly suggest that UGT1A1 is responsible for G2 formation in human liver. In contrast, no such correlation was observed with G3 formation, suggesting that multiple UGT isoforms, including UGT1A1 and UGT1A3, are involved in G3 formation. G2 is also observed in rat and monkey liver microsomes as a major metabolite of T-5224, suggesting that G2 is not a human-specific metabolite. In this study, we obtained useful information on the metabolism of T-5224 for its clinical use.[5] Background: Sepsis has been identified as the most common cause of acute kidney injury (AKI) in intensive care units. Lipopolysaccharide (LPS) induces the production of several proinflammatory cytokines including tumor necrosis factor (TNF)-alpha, a major pathogenetic factor in septic AKI. c-Fos/activator protein (AP)-1 controls the expression of these cytokines by binding directly to AP-1 motifs in the cytokine promoter regions. T-5224 is a new drug developed by computer-aided drug design that selectively inhibits c-Fos/AP-1 binding to DNA. In this study, we tested whether T-5224 has a potential inhibitory effect against LPS-induced AKI, by suppressing the TNF-alpha inflammatory response and other downstream effectors.[6] Methods: To test this hypothesis, male C57BL/6 mice at 7 weeks old were divided into three groups (control, LPS and T-5224 groups). Mice in the control group received saline intraperitoneally and polyvinylpyrrolidone solution orally. Mice in the LPS group were injected intraperitoneally with a 6 mg/kg dose of LPS and were given polyvinylpyrrolidone solution immediately after LPS injection. In the T-5224 group, mice were administered T-5224 orally at a dose of 300 mg/kg immediately after LPS injection. Serum concentrations of TNF-alpha, interleukin (IL)-1beta, IL-6 and IL-10 were measured by ELISA. Moreover, the expression of intercellular adhesion molecule (ICAM)-1 mRNA in kidney was examined by quantitative real-time RT-PCR. Finally, we evaluated renal histological changes.[6] Results: LPS injection induced high serum levels of TNF-alpha, IL-1beta and IL-6. However, the administration of T-5224 inhibited the LPS-induced increase in these cytokine levels. The serum levels of IL-10 in the LPS group and T-5224 group were markedly elevated compared with the control group. T-5224 also inhibited LPS-induced ICAM-1 mRNA expression. Furthermore histological studies supported an anti-inflammatory role of T-5224.[6] Conclusions: In endotoxin-induced AKI, T-5224 inhibited the production of TNF-alpha and other downstream effectors. In contrast, T-5224 did not inhibit IL-10, an anti-inflammatory cytokine. These data support that the use of T-5224 is a promising new treatment for septic kidney injury.[6] |
| 分子式 |
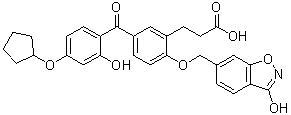
C29H27NO8
|
|
|---|---|---|
| 分子量 |
517.53
|
|
| 精确质量 |
517.173
|
|
| 元素分析 |
C, 67.30; H, 5.26; N, 2.71; O, 24.73
|
|
| CAS号 |
530141-72-1
|
|
| 相关CAS号 |
|
|
| PubChem CID |
23626877
|
|
| 外观&性状 |
Typically exists as White to yellow solids at room temperature
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
774.1±60.0 °C at 760 mmHg
|
|
| 闪点 |
422.0±32.9 °C
|
|
| 蒸汽压 |
0.0±2.8 mmHg at 25°C
|
|
| 折射率 |
1.665
|
|
| LogP |
5.14
|
|
| tPSA |
139.32
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
38
|
|
| 分子复杂度/Complexity |
844
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O(C1C([H])=C([H])C(C(C2C([H])=C([H])C(=C(C=2[H])C([H])([H])C([H])([H])C(=O)O[H])OC([H])([H])C2C([H])=C([H])C3C(N([H])OC=3C=2[H])=O)=O)=C(C=1[H])O[H])C1([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H]
|
|
| InChi Key |
DALCQQSLNPLQFZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C29H27NO8/c31-24-15-21(37-20-3-1-2-4-20)8-10-22(24)28(34)19-6-11-25(18(14-19)7-12-27(32)33)36-16-17-5-9-23-26(13-17)38-30-29(23)35/h5-6,8-11,13-15,20,31H,1-4,7,12,16H2,(H,30,35)(H,32,33)
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (9.66 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 50.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 5 mg/mL (9.66 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9323 mL | 9.6613 mL | 19.3226 mL | |
| 5 mM | 0.3865 mL | 1.9323 mL | 3.8645 mL | |
| 10 mM | 0.1932 mL | 0.9661 mL | 1.9323 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Effect of T‐5224 on tumor cell proliferationin vitro.Cancer Sci.2016 May;107(5):666-73. |
|---|
 Protocol and results ofin vivostudy using an orthotopic model of head and neck squamous cell carcinoma.
Morphological changes in tumor cells (HSC‐3‐M3 head and neck squamous cell carcinoma) after replacement with normal or T‐5224 (+) media.Cancer Sci.2016 May;107(5):666-73. |
 Effect of T‐5224 on the transcription and activity of MMP‐2 and ‐9.
Effect of T‐5224 on the invasion activity of HSC‐3‐M3 head and neck squamous cell carcinoma cells.Cancer Sci.2016 May;107(5):666-73. |