| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
TMEM16A/transmembrane protein 16A
|
|---|---|
| 体外研究 (In Vitro) |
在不显着影响 L 型 Ca2+ 电流的情况下,T16Ainh-A01 (0.3-30 μM) 大大降低了 IClCa 的内向和外向成分,并抑制 RUICC 中的 IClCa [1]。在所有电压下,TMEM16A 氯化物电流(由移液器中 275 nM 游离钙引起)几乎完全被 T16Ainh-A01 (10 μM) 抑制,表明存在电压无关的阻断机制 [2]。
|
| 体内研究 (In Vivo) |
从兔尿道分离的Cajal间质细胞(ICC)表现出Ca2+激活的Cl-电流(I ClCa),这对尿道张力的发展很重要。在这里,我们通过检查“新一代”TMEM16A抑制剂CACCinh-A01和T16Ainh-A01对从新鲜分离的兔尿道ICC(RUICC)记录的I ClCa和对完整兔尿道平滑肌条收缩的影响,来检查TMEM16A(ANO1)是否对此活动有贡献。实时定量PCR实验表明,与TMEM16B和TMEM16F相比,TMEM16A在兔尿道平滑肌中高度表达。单细胞RT-PCR实验表明,新鲜分离的RUICC中仅表达TMEM16A。CACCinh-A01和T16Ainh-A01抑制了用电压钳记录的离体RUICC中去极化诱发的I ClCa,IC50值分别为1.2和3.4μM。同样,CACCinh-A01和T16Ainh-A01也抑制了从-60 mV箝位的RUICC电压中记录的自发瞬态内向电流(STIC)和电流钳中记录的自发性瞬态去极化(STD)。相比之下,CACCinh-A01和T16Ainh-A01仅部分降低了孤立的RUICC中的自发Ca2+波。最后,CACCinh-A01和T16Ainh-A01也显著减少了电场刺激(EFS)引起的兔尿道平滑肌条(RUSM)的神经源性收缩。这些数据与TMEM16A参与RUICC和兔尿道平滑肌收缩的CACCs的观点一致[1]。
|
| 酶活实验 |
筛选过程[2]
如所述,使用配备FluoStar荧光板阅读器的自动筛选平台(Beckman)进行高通量筛选。96孔板的每个孔用PBS(200μl/次洗涤)洗涤三次,留下50μl PBS。将试验化合物(0.5μl)以25μm的终浓度加入每个孔中。10分钟后,将96孔板转移到读板器上进行荧光测定。通过连续记录荧光(400 ms/点)2 s(基线),分别测定每个孔的TMEM16A介导的I-内流,然后加入50μl含有200μm ATP的140 mm I-溶液。I−流入的初始速率是通过非线性回归从荧光数据中计算出来的。[2] 短路电流[2] 将含有表达TMEM16A的FRT细胞、T84细胞或人支气管上皮细胞的Snapwell插入物安装在Ussing室中。将阿米洛利、CFTRinh-172、UTP、ATP和TMEM16A抑制剂加入根尖溶液中,同时将等体积的载体加入基底外侧溶液中。对称的HCO3-缓冲溶液用于T84细胞和人支气管上皮细胞。对于FRT细胞,半腔充满了半Cl-溶液(顶端)和HCO3-缓冲溶液(基底外侧)。将细胞浸泡10分钟稳定期,并在37°C或室温下用95%O2/5%CO2充气。在一些实验中,为了测量根尖氯离子电导率,用制霉菌素(360μg/ml)渗透基底侧膜,并施加氯离子梯度,其中基底侧膜用HCO3-缓冲溶液浸泡,在根尖溶液中用葡萄糖酸钠代替120mm NaCl。使用EVC4000多通道V/I箝位器(佛罗里达州萨拉索塔市世界精密仪器公司)测量短路电流,并使用PowerLab/8sp记录。[2] 贴片夹[2] 在室温下对表达TMEM16A的FRT细胞和人颌下腺A253细胞进行全细胞记录。移液管溶液含有130 mm CsCl、0.5 mm EGTA、1 mm MgCl2、1 mm Tris-ATP和10 mm HEPES(pH 7.2)。浴溶液含有140毫米N-甲基-d-葡糖胺-Cl、1毫米CaCl2、1毫米MgCl2、10毫米葡萄糖和10毫米HEPES(pH 7.4)。移液管由硼硅酸盐玻璃制成,经过火抛光后电阻为3-5兆欧。密封电阻在3到10千欧姆之间。在建立全细胞结构后,TMEM16A被100μm ATP或移液管溶液中275nm游离钙(向移液管液中加入1mm CaCl2)激活。通过施加超极化和去极化电压脉冲,从0 mV的保持电位到-100 mV至+100 mV的电位,以20 mV的步幅,引发全细胞电流。在室温下使用Axopatch-200B进行记录。电流用Digidata 1440A转换器数字化,以5kHz滤波,以1kHz采样。[2] 细胞质钙测量[2] 按照制造商的方案,在镀覆后48小时,将96孔黑壁微孔板中的FRT细胞装载Fluo-4 NW。使用配备注射泵和定制激发/发射滤光片(485/538nm)的FLUOstar Optima荧光板阅读器测量Fluo-4荧光。 |
| 细胞实验 |
全细胞膜片钳记录[1]
采用全细胞膜片钳技术的穿孔贴片配置记录电流。这避免了使用传统全电池配置时遇到的电流耗尽问题。使用抗生素两性霉素B(600μg/ml)穿孔细胞膜。贴片移液管最初通过浸入移液管溶液进行前填充,然后用含两性霉素B的溶液进行后填充。移液管从硼硅酸盐玻璃毛细管(外径1.5 mm,内径1.17 mm)中拔出,尖端直径约为1-1.5μm,电阻为2-4 mΩ。电压钳位命令通过Axopatch 1D膜片钳放大器传递,该放大器连接到与运行pClamp软件的计算机连接的Digidata 1440A AD/DA转换器。在实验过程中,通过一个由移液管(尖端直径200μm)组成的紧密输送系统,将Hanks溶液持续地注入所研究的细胞,该移液管放置在约300μm远的地方。这可以在死区时间约为5秒的情况下切换到含有药物的溶液。所有实验均在35-37°C下进行。[1] 钙显像[1] 将细胞置于含有100μM Ca2+的Hanks溶液中,并让其在玻璃底培养皿中沉淀,直到它们粘在一起。然后将它们在室温下在黑暗中在0.4μM fluo-4/AM中孵育6-8分钟,然后在37°C下进行研究。如前所述,使用iXon 887 EMCCD相机(512×512像素,像素尺寸16×16μm)与Nipkow旋转盘共聚焦头连接对细胞进行成像。使用488nm的氪氩激光器激发fluo-4,在波长>510nm处检测到发射的光。使用×60物镜(奥林巴斯)进行实验,得到像素尺寸为0.266×0.266μm的图像。以每秒15帧的速度采集图像。从每一帧中减去使用零帧获得的相机背景荧光,以获得“F”。F 0被确定为在控制条件下振荡之间测量的最小荧光。为了获得用于在图形中显示的事后行扫描图像,在整个单元格的中心绘制了一条一像素厚的线,并调用了ImageJ中的“reslice”命令。图8a显示了一个例子,其中显示了在自发Ca2+波期间拍摄的RUCC电影堆栈中的选定帧。第一帧中显示的白线表示获得图8b所示伪线扫描的区域。因此,重要的是要认识到,伪线扫描图像仅表示沿线长度发生的事件,而不包括发生在该区域之外的事件。∆F/F 0是指测量Ca2+水平从基础到峰值的变化。 |
| 动物实验 |
Male and female New Zealand white rabbits (16–20 weeks old, 2.5–4 kg weight) were humanely killed with a lethal injection of pentobarbitone (i.v.). The most proximal 1.5 cm of the urethra was removed and placed in Krebs’ solution and individual ICC were isolated enzymatically as described previously (Sergeant et al. 2000).[2]
Dissected strips of rabbit urethra smooth muscle were stored for subsequent use at −20 °C in RNAlater. Immediately prior to isolation of the RNA, tissue samples were transferred to a 1.5-ml tube, snap frozen in liquid nitrogen, and pulverized to yield a dry powder. RNA was isolated from these samples using the RNeasy mini kit. All samples were DNase treated and the purified RNA was eluted with RNase free water. After determination of the RNA concentration using a nanodrop spectrophotometer, the purified RNA was stored at −80 °C. Prior to cDNA synthesis, RNA was denatured for 5 min at 70 °C and then rapidly cooled on ice. RNA was reverse transcribed using the Superscript VILO cDNA synthesis kit according to the manufacturer’s instructions. Rabbit brain tissue was processed in parallel for use as a control, and cDNA was generated from brain-derived RNA as described above.[2] Real-time quantitative PCR (qPCR) was performed using the SYBR Green PCR Master Mix. TMEM16B and F gene-specific primer sets were designed to span exon-exon boundaries present in the known TMEM16 transcripts. TMEM16A primers were designed from 5′ sequence isolated in our laboratory. β-actin was used in qPCR as an endogenous reference gene for sample normalization, and a β-actin primer set was designed accordingly. [2] |
| 参考文献 |
|
| 其他信息 |
TMEM16A (ANO1) functions as a calcium-activated chloride channel (CaCC). We developed pharmacological tools to investigate the contribution of TMEM16A to CaCC conductance in human airway and intestinal epithelial cells. A screen of ∼110,000 compounds revealed four novel chemical classes of small molecule TMEM16A inhibitors that fully blocked TMEM16A chloride current with an IC(50) < 10 μM, without interfering with calcium signaling. Following structure-activity analysis, the most potent inhibitor, an aminophenylthiazole (T16A(inh)-A01), had an IC(50) of ∼1 μM. Two distinct types of inhibitors were identified. Some compounds, such as tannic acid and the arylaminothiophene CaCC(inh)-A01, fully inhibited CaCC current in human bronchial and intestinal cells. Other compounds, including T16A(inh)-A01 and digallic acid, inhibited total CaCC current in these cells poorly, but blocked mainly an initial, agonist-stimulated transient chloride current. TMEM16A RNAi knockdown also inhibited mainly the transient chloride current. In contrast to the airway and intestinal cells, all TMEM16A inhibitors fully blocked CaCC current in salivary gland cells. We conclude that TMEM16A carries nearly all CaCC current in salivary gland epithelium, but is a minor contributor to total CaCC current in airway and intestinal epithelia. The small molecule inhibitors identified here permit pharmacological dissection of TMEM16A/CaCC function and are potential development candidates for drug therapy of hypertension, pain, diarrhea, and excessive mucus production.[2]
|
| 分子式 |
C19H20N4O3S2
|
|---|---|
| 分子量 |
416.5171
|
| 精确质量 |
416.098
|
| 元素分析 |
C, 54.79; H, 4.84; N, 13.45; O, 11.52; S, 15.39
|
| CAS号 |
552309-42-9
|
| PubChem CID |
135460621
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.565
|
| tPSA |
154.26
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
660
|
| 定义原子立体中心数目 |
0
|
| SMILES |
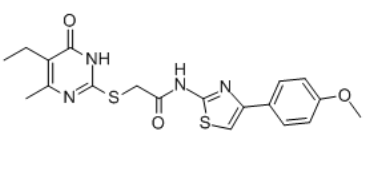
CCC1=C(N=C(SCC(NC2=NC(C3=CC=C(OC)C=C3)=CS2)=O)N=C1O)C
|
| InChi Key |
QSIYTNYMBWYHAA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H20N4O3S2/c1-4-14-11(2)20-18(23-17(14)25)28-10-16(24)22-19-21-15(9-27-19)12-5-7-13(26-3)8-6-12/h5-9H,4,10H2,1-3H3,(H,20,23,25)(H,21,22,24)
|
| 化学名 |
2-[(5-ethyl-4-methyl-6-oxo-1H-pyrimidin-2-yl)sulfanyl]-N-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl]acetamide
|
| 别名 |
T16Ainh-A01; T16Ainh A01; T16AInh-A01; 2-((5-Ethyl-4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(4-methoxyphenyl)thiazol-2-yl)acetamide; 2-[(5-Ethyl-1,6-dihydro-4-methyl-6-oxo-2-pyrimidinyl)thio]-N-[4-(4-methoxyphenyl)-2-thiazolyl]acetamide; T16Ainh - A01; T16Ainh-A01;; t16a(inh)-a01; 2-[(5-ethyl-6-methyl-4-oxo-1H-pyrimidin-2-yl)sulfanyl]-N-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl]acetamide; T16Ainh-A-01
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~83.33 mg/mL (~200.06 mM)
DMF :≥ 10 mg/mL (~24.01 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.99 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4008 mL | 12.0042 mL | 24.0085 mL | |
| 5 mM | 0.4802 mL | 2.4008 mL | 4.8017 mL | |
| 10 mM | 0.2401 mL | 1.2004 mL | 2.4008 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。