| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
GABA receptor
|
|---|---|
| 体外研究 (In Vitro) |
在 2/3 层神经元中,THIP(1 µM;5 s)产生强 GABAA 介导的电流 [1]。在第 2/3 层神经元中,Gaboxadol/THIP(1 µM;1 s)对 micro-IPSC 没有影响 [1]。
THIP/Gaboxadol(4,5,6,7-四氢异恶唑[5,4-c]吡啶-3-醇)是一种选择性GABA(a)受体激动剂,偏爱含有GABA(a)受体的δ亚基。THIP目前正在人体试验中测试其催眠效果,显示出有利的耐受性和成瘾性。由于THIP在新皮层中的细胞作用尚不确定,我们研究了THIP对13-19日龄(P13-19)小鼠额顶叶新皮层切片神经元的影响。通过全细胞膜片钳记录,我们发现临床相关的1mum THIP浓度在2/3层神经元中诱导了强劲的GABA(a)介导的强直电流。相比之下,通过模拟体内内源性GABA水平,只诱导了一分钟的紧张电流。微型IPSCs不受1mum THIP的影响,提示其作用位点位于突触外。THIP的EC(50)为44 μ m。THIP诱导的第2/3层神经元的强压电流比第5层神经元的强压电流大44%,与新皮层浅层含有δ受体的表达增强一致。最后,通过监测自发活动的新皮层神经元,THIP引起抑制活性的整体抑制,同时显著增强兴奋性活性。我们的研究表明,THIP激活了突触外GABA(A)受体介导的新皮层传导,这可能会改变皮层网络的活性。[1] Gaboxadol/加博沙多 转运通过Caco-2细胞单层的表征[2] 在不同的加博沙多浓度、顶端pH值以及缺乏或存在5-HTP的情况下,研究了加博沙多在Caco-2细胞单层上的经上皮转运(表1)。在质子梯度穿过单层的情况下(顶端和基底侧腔的pH值分别为6.0和7.4),加博沙多的平均a - b通量增加,而加博沙多的渗透率分别从8.0 × 10 - 6下降到6.1 × 10 - 6和5.6 × 10 - 6 cm s - 1 (p < 0.05),分别为0.34、3.5和7.0 mM加博沙多(表1)。因此,加博沙多在Caco-2细胞中的渗透性是浓度依赖性的,这与hPAT1的部分饱和是一致的。在没有质子梯度的情况下(顶端和基底侧腔的pH值分别为7.4和7.4),3.5 mM和7 mM加博沙多的渗透性分别降低了约5倍和7倍(p < 0.01和p < 0.001)。这与加博沙多通过hPAT1转运是一致的,因为转运体是质子偶联的。在5-HTP存在的情况下,加博沙多的通透性降低了约5倍(p < 0.001)。加博沙多在B-A方向的转运是A-B方向转运的21%,因此加博沙多在A-B方向上的转运是极化的(p < 0.01)。加博沙多在没有质子梯度或有5-HTP存在时的渗透率与加博沙多在B-A方向的渗透率和[14C]-甘露醇的渗透率非常相似。总之,这些观察结果表明,质子依赖的氨基酸转运蛋白hPAT1介导了加博沙多在肠细胞刷状边界膜上的转运。此外,结果表明hPAT1在Caco-2细胞单层中负责80-90%的加博沙多经上皮转运。 盐酸加波沙朵/Gaboxadol(0.34、3.5和7.0 μM)以剂量依赖性方式降低Caco-2单层细胞的通透性,平均Papp值为8.1 × 10-6 cm·s-1、6.1 × 10 -1 cm·s-1(0.34、3.5 和 7 μM 加波沙朵),5.6 × 10-6 cm·s-1(0.34、3.5 和 7 μM 加波沙朵)[6]。 Gaboxadol在体外通过hPAT1转运的特性 [6] 在Gaboxadol/加博沙多浓度增加的情况下,通过测量hPAT1底物脯氨酸进入cco -2细胞单层的顶端摄取,研究了加博沙多和hPAT1之间的相互作用(图1)。加博沙多降低cco -2细胞单层的顶端脯氨酸摄取,估计抑制剂亲和力(Ki值)为6.6 mmol·L−1。同样,已知的PAT1抑制剂色氨酸也降低了脯氨酸的顶端吸收,Ki值为7.7 mmol·L−1。研究了三种浓度(0.34、3.5和7.0 mmol·L−1)下加博沙多在caco2细胞单层上的经上皮转运(A-B)通量。对于0.34、3.5和7 mmol·L−1的加博沙多,加博沙多转运的平均Papp值分别为8.1 × 10−6 cm·s−1、6.1 × 10−6 cm·s−1和5.6 × 10−6 cm·s−1(图2)。因此,随着加博沙多浓度的增加,加博沙多通过Caco-2细胞单层的通透性降低(P < 0.05)。在35 mmol·L−1色氨酸存在的情况下,研究了加博沙多在caco2细胞单层中使用3.5 mmol·L−1加博沙多的顶端浓度,有或没有pH梯度,双向运输(图2)。加博沙多在A-B方向的转运量约为B-A方向的5倍(P < 0.005)。色氨酸的存在使加博沙多的通透性降低了53% (Papp为2.9 × 10−6 cm·s−1,P < 0.005)。在没有质子梯度的情况下,加博沙多的渗透率降低了82%,为1.1 × 10−6 cm·s−1 (P < 0.005)。在色氨酸存在、质子梯度不存在的情况下,加博沙多在B-A方向的渗透性与[3H]-甘露醇的渗透性相似(Papp为1.6±0.36 × 10−6 cm·s−1)。此外,在转运实验中,加博沙多和色氨酸的存在并没有改变美托洛尔或甘露醇在Caco-2细胞单层间转运的通透性。甘露醇和美托洛尔的渗透率分别为1.6±0.36 × 10−6 cm·s−1和6.9±0.99 × 10−6 cm·s−1。综上所述,加博沙多通过Caco-2细胞单层的上皮转运是ph依赖的,可以被色氨酸抑制,并在A-B方向极化。综上所述,这些观察结果表明,hPAT1介导了加博沙多在人肠上皮细胞腔膜上的转运,这一转运步骤在很大程度上决定了加博沙多的上皮转运。 加博沙多/Gaboxadol转运通过hPAT1体外的表征[6] 在加博沙多浓度增加的情况下,通过测量hPAT1底物脯氨酸进入cco -2细胞单层的顶端摄取,研究了加博沙多和hPAT1之间的相互作用(图1)。加博沙多降低cco -2细胞单层的顶端脯氨酸摄取,估计抑制剂亲和力(Ki值)为6.6 mmol·L−1。同样,已知的PAT1抑制剂色氨酸也降低了脯氨酸的顶端吸收,Ki值为7.7 mmol·L−1。研究了三种浓度(0.34、3.5和7.0 mmol·L−1)下加博沙多在caco2细胞单层上的经上皮转运(A-B)通量。对于0.34、3.5和7 mmol·L−1的加博沙多,加博沙多转运的平均Papp值分别为8.1 × 10−6 cm·s−1、6.1 × 10−6 cm·s−1和5.6 × 10−6 cm·s−1(图2)。因此,随着加博沙多浓度的增加,加博沙多通过Caco-2细胞单层的通透性降低(P < 0.05)。在35 mmol·L−1色氨酸存在的情况下,研究了加博沙多在caco2细胞单层中使用3.5 mmol·L−1加博沙多的顶端浓度,有或没有pH梯度,双向运输(图2)。加博沙多在A-B方向的转运量约为B-A方向的5倍(P < 0.005)。色氨酸的存在使加博沙多的通透性降低了53% (Papp为2.9 × 10−6 cm·s−1,P < 0.005)。在没有质子梯度的情况下,加博沙多的渗透率降低了82%,为1.1 × 10−6 cm·s−1 (P < 0.005)。在色氨酸存在、质子梯度不存在的情况下,加博沙多在B-A方向的渗透性与[3H]-甘露醇的渗透性相似(Papp为1.6±0.36 × 10−6 cm·s−1)。此外,在转运实验中,加博沙多和色氨酸的存在并没有改变美托洛尔或甘露醇在Caco-2细胞单层间转运的通透性。甘露醇和美托洛尔的渗透率分别为1.6±0.36 × 10−6 cm·s−1和6.9±0.99 × 10−6 cm·s−1。综上所述,加博沙多通过Caco-2细胞单层的上皮转运是ph依赖的,可以被色氨酸抑制,并在A-B方向极化。综上所述,这些观察结果表明,hPAT1介导了加博沙多在人肠上皮细胞腔膜上的转运,这一转运步骤在很大程度上决定了加博沙多的上皮转运。 Gaboxadol是Caco-2细胞单层中质子偶联氨基酸转运体hPAT1的底物[6] 加博沙多抑制cco -2细胞对hPAT1底物脯氨酸的顶端吸收,Ki值为6.6 mmol·L−1。这种亲和力与最近观察到的其他hPAT1底物如GABA (3.1 mmol·L−1)和GABA类似物muscimol (1.7 mmol·L−1)和THPO (11.3 mmol·L−1)的亲和力相当(Larsen et al., 2008)。色氨酸的亲和力与加博沙多相当,为7.7 mmol·L−1。Metzner等人(2005)先前将色氨酸描述为PAT1的抑制剂,并报道了cco -2细胞中通过hPAT1摄取脯氨酸的Ki值为4.7 mmol·L−1。考虑到两个实验室对hPAT1脯氨酸亲和力的微小差异;Metzner等人报告的Kt值为1.4 mmol·L−1,而Larsen等人报告的Km值分别为3.6 mmol·L−1,两项研究中色氨酸对hPAT1的亲和力相当(Metzner等人,2005;Larsen et al., 2008)。根据其他小组发表的结果(Thwaites et al., 1993;Metzner et al., 2005),我们发现ccao -2细胞的大部分顶端脯氨酸运输是由hPAT1介导的,没有观察到脯氨酸的其他钠依赖性或钠依赖性转运体的证据(Larsen et al., 2008)。其他氨基酸转运蛋白,如顶钠依赖性氨基酸转运蛋白B0 (B0AT1), B0,+ (ATB0,+)和系统(ASC) (ASCT2)不太可能参与加博沙多的转运。它们都存在于Caco-2细胞中,但它们的底物移位不是质子偶联的。此外,与PAT1相比,这些转运蛋白对底物的亲和力值更高,例如ASC (ASCT2),约为100 μ mol·L−1 (Uchiyama et al., 2005);B0 (B0AT1), 500-700µmol·L−1 (Broer et al., 2004)和B0,+ (ATB0,+),约150µmol·L−1 (Hatanaka et al., 2002)。 Gaboxadol在Caco-2细胞单层间的上皮运输呈尖向基底侧极化。色氨酸可以抑制加博沙多的转运,并依赖于顶端供体溶液的pH。此外,随着加博沙多浓度的增加,加博沙多在根尖-基底侧方向的Papp降低。这与加博沙多通过hPAT1转运一致,该途径约占总上皮转运的80%。在小鼠小肠和Caco-2细胞中发现了氨基酸转运系统b0,+,分别占丙氨酸和精氨酸总转运量的15-85% (Wenzel et al., 2001;Dave et al., 2004)。然而,色氨酸与系统b0 +的结合尚未明确显示(Su et al., 1992;Tate et al., 1992),此外,阳离子氨基酸、两性离子氨基酸和胱氨酸对体系b0,+具有µmol·L−1的亲和性(Palacin, 1994)。因此,如果加博沙多通过系统b0,+运输到任何显著程度,它在Caco-2细胞中应该是明显的。在体外,经上皮的加博沙多转运还具有ph依赖性,而PAT1是目前已知的肠道中唯一的质子偶联氨基酸转运蛋白。加巴喷丁(也是两性离子γ-氨基类似物)在大鼠小肠中的渗透性被证明是质子独立的(Nguyen et al., 2007),因此加巴喷丁和加博沙多存在不同的根尖转运机制。结果表明,加博沙多是Caco-2细胞单层中hPAT1的底物,hPAT1介导加博沙多通过肠道肠细胞的管腔膜运输,这似乎对由此产生的上皮运输很重要。加博沙多跨基底外膜外排的机制尚不清楚。 加博沙多/Gaboxadol的体内吸收 在狗[6] 口服给药后,加博沙多在犬体内吸收迅速,Tmax约为0.46 h,生物利用度高达85%。这些观察结果与先前关于人体口服吸收的研究一致,表明加博沙多的Tmax约为0.5 h,生物利用度约为90% (Schultz等人,1981;Lund et al., 2006)。一旦被吸收,加博沙多主要以加博沙多的形式从尿液中排出,而一小部分以葡萄糖醛酸偶联物的形式排出,在大鼠和小鼠中占2-7%,在两个人类受试者中占30-35% (Schultz et al., 1981;Lund et al., 2006;Shadle et al., 2006)。总的来说,这表明在狗体内,加博沙多被迅速和完全吸收,可能在小肠近端,吸收后代谢最小。 |
| 体内研究 (In Vivo) |
加波沙朵(THIP;0.5、5.0 mg/kg;口服;单剂量)表现出良好的口服利用度;在 0.5 和 5.0 mg/kg 时,Fa 值分别为 83% 和 110% [2]。
研究了大鼠口服加博沙多/Gaboxadol在预剂量5-HTP存在和不存在的情况下的体内药代动力学特征。在Caco-2细胞单层>中,80%的吸收性加博沙多转运被认为是hpat1介导的。在大鼠体内,加博沙多的初始吸收率在5-羟色胺的存在下降低。5-羟色胺预给药后,加博沙多的AUC增加3.6 ~ 5.5倍。加博沙多是肾转运蛋白rOat1的底物,K(m)值为151微米。5- http不与rOat1相互作用。综上所述,加博沙多作为hPAT1的底物,也是rOat1的底物。在大鼠中,5-HTP降低了加博沙多的初始吸收率,增加了AUC。因此,5-HTP对加博沙多的药代动力学有显著影响。[2] 盐酸加波沙朵/Gaboxadol(腹膜内注射;0.5、1、1.5、2、3、4 或 5 mg/kg;每天三次;间隔三天)使 Fmr1 KO2 小鼠的步行距离正常化至 0.5 mg/kg WT 活动水平,此外,该化学物质对 Fmr1 KO2 小鼠的运动活性没有影响 [5]。 在这里,我们试图评估Gaboxadol(也称为OV101和THIP),一种选择性和有效的含有δ亚基的突触外GABAA受体(dSEGA)的激动剂,通过评估其在相对未表征的FXS小鼠模型(Fmr1 KO2小鼠)中的异常行为正常化能力,作为FXS的治疗剂。四个行为领域(多动、焦虑、攻击和重复行为)通过一系列行为分析进行了探讨。结果显示,与野生型(WT)相比,Fmr1 KO2小鼠过度活跃,具有异常焦虑样行为,更易怒和更具攻击性,重复行为频率增加,这些都是FXS个体的行为缺陷。加博沙多/Gaboxadol治疗使Fmr1 KO2小鼠中观察到的所有异常行为恢复到WT水平,为其治疗FXS的潜在益处提供了证据。我们发现,仅加博沙多就能增强突触外GABA受体,足以使FXS模型中的许多行为缺陷正常化,这些缺陷的终点可直接转化为FXS的临床表现。综上所述,这些数据支持未来对FXS患者加博沙多的评估,特别是关于多动症、焦虑、易怒、攻击和重复行为的症状。[5] 加博沙多使Fmr1 KO2小鼠的过度活动正常化[5] 多动是人类FXS的一个显著特征(Bailey et al., 2008;Wheeler et al., 2014;Hagerman et al., 2017),并已在先前表征的荷兰-比利时Fmr1 KO小鼠中可靠地复制(Olmos-Serrano et al., 2010;Kazdoba et al., 2014)。为了测试Fmr1 KO2小鼠是否表现出运动亢进,以及加博沙多是否能使这种异常行为正常化,我们给Fmr1 KO2小鼠注射了载药或加博沙多(0.5-5 mg/kg, i.p),并在OFT测试前30分钟给WT窝仔注射载药。记录30 min在OFT内行走的总距离(cm)。结果显示,Fmr1 KO2小鼠的行走距离较WT窝中对照组显著增加(图1,F(8,81) = 21.27, p < 0.0001),与其他FXS模型的结果一致。加博沙多(0.5 mg/kg)使Fmr1 KO2小鼠的行走距离正常化到WT活性水平(图1)。高剂量的加博沙多(1 - 5 mg/kg, ig)对Fmr1 KO2小鼠的运动活性没有影响(图1)。这些结果不能归因于加博沙多的镇静作用,因为在WT C57Bl/6或BALB/c小鼠中,加博沙多高达2.0 mg/kg的剂量在60分钟的OFT中对运动活动没有影响(数据未显示),这与先前的研究一致,表明加博沙多对WT小鼠(Olmos-Serrano等人,2011)或大鼠(Silverman等人,2016)的运动没有影响。 Fmr1 KO2小鼠焦虑样行为经加博沙多归一化 [5] 为了评估加博沙多对Fmr1 KO2小鼠焦虑样行为的影响,采用了三种不同的行为测试:OFT、LDT和SAT的中心移动距离。中心移动距离的增加被解释为焦虑的减少,并利用了小鼠在进入新环境时保持在周边的固有偏好。Fmr1 KO2小鼠注射加博沙多(0.5-5 mg/kg, i.p), WT窝仔在放置于OFT中30分钟前注射载药。与WT对照组相比,Fmr1 KO2小鼠在中心行走的总距离显著增加(图2A, F(8,81) = 21.32, p < 0.0001)。加博沙多治疗(0.5 mg/kg, i.p.p)使Fmr1 KO2对中心距离的影响正常化,达到与WT对照组相当的水平(图2A)。在本实验中,高剂量的加博沙多(1-5 mg/kg)对Fmr1 KO2小鼠没有影响(图2A)。 加博沙多使Fmr1 KO2小鼠的易怒和攻击行为正常化 [5] 与其他形式的综合征自闭症一样,很大一部分FXS患者表现出易怒、社交焦虑和攻击性。这些异常行为可以在啮齿类动物中建模,通过表征测试小鼠和新笼伴侣之间的si。为了验证Fmr1 KO2突变体易怒和攻击性增加的假设,我们量化了摇尾、咬人行为、攀爬行为和攻击延迟的实例。小鼠入笼前30 min分别注射载药或加博沙多(0.5 ~ 5mg /kg, i.p)。 尾巴嘎嘎作响,或尾巴的快速振动,反映了攻击性和战斗倾向。与WT对照组相比,Fmr1 KO2小鼠的摇尾频率显著增加(图3A, F(8,81) = 16.03, p < 0.0001)。加博沙多(0.5、1.5和5.0 mg/kg)使Fmr1 KO2小鼠的效果正常化,达到与WT对照组相当的水平(图3A)。 Gaboxadol使Fmr1 KO2小鼠的重复行为正常化[5] 坚持和重复行为在FXS患者中很常见,并且具有高度破坏性(Arron et al., 2011;Leekam et al., 2011;Hall et al., 2016)。为了验证这些特征可能在Fmr1 KO2动物中观察到的假设,我们量化了WT和Fmr1 KO2突变小鼠的打圈、自我梳理和刻板印象。小鼠分别注射载药或加博沙多(0.5 ~ 5mg /kg, i.p)后,在实验室内测量逆时针转数(CCW)。与WT对照组相比,Fmr1 KO2小鼠在5分钟测试期间CCW转数显著增加(图4A, F(8,81) = 25.46, p < 0.0001)。向Fmr1 KO2小鼠注射加博沙多(0.5、1.0 mg/kg)后,CCW转数恢复到WT水平(图4A)。基因型对顺时针旋转无影响(p = 0.386,数据未显示)。 加博沙多的体内吸收 在狗[6] 口服给药后,加博沙多在犬体内吸收迅速,Tmax约为0.46 h,生物利用度高达85%。这些观察结果与先前关于人体口服吸收的研究一致,表明加博沙多的Tmax约为0.5 h,生物利用度约为90% (Schultz等人,1981;Lund et al., 2006)。一旦被吸收,加博沙多主要以加博沙多的形式从尿液中排出,而一小部分以葡萄糖醛酸偶联物的形式排出,在大鼠和小鼠中占2-7%,在两个人类受试者中占30-35% (Schultz et al., 1981;Lund et al., 2006;Shadle et al., 2006)。总的来说,这表明在狗体内,加博沙多被迅速和完全吸收,可能在小肠近端,吸收后代谢最小。 联合给药色氨酸后,Gaboxadol/加博沙多的体内吸收[6] 同时给药hPAT1抑制剂色氨酸对加博沙多的吸收谱有剂量依赖性,导致Cmax降低和Tmax增加。加博沙多吸收率的降低可由胃排空率的改变引起。在人类中,胃排空的速度随着一餐所摄入的卡路里数量的增加而降低(Calbet和MacLean, 1997;Sunesen et al., 2005),在狗的实验中,膳食成分也被证明可以延长胃排空(Mizuta et al., 1990)。为了排除观察到的对加博沙多吸收的影响是胃排空改变的结果,研究了常被用作胃排空标志的扑热息痛的累积吸收曲线(Calbet and MacLean, 1997;Sunesen et al., 2005),在色氨酸存在的情况下进行了研究。高剂量色氨酸对扑热息痛的胃排空无显著影响。然而,色氨酸对加博沙多的ka有显著影响。由于共给色氨酸改变了加博沙多Tmax, Cmax和ka,而Fa, ke和AUC不变,色氨酸的作用可能是色氨酸和加博沙多在吸收部位相互作用的结果,而不是由于胃排空的改变。其他研究表明,加博沙多与血浆蛋白结合程度较低,不被细胞色素p -450代谢(Lund et al., 2006)。因此,根据体外实验结果表明,hPAT1介导了Caco-2单层中大部分加博沙多的腔内转运,似乎体内观察也可以解释为pat1介导的狗对加博沙多的吸收,而这种吸收被色氨酸的共同给药所减少。从色氨酸对加博沙多Cmax的影响或对肠道吸收速率常数ka的影响来估计色氨酸抑制加博沙多肠道运输的体内亲和值。IC50值分别为10.1和12.6 mmol·L−1。如前所述,在Caco-2细胞中,hPAT1对色氨酸的体外亲和力(通过hPAT1抑制脯氨酸运输)为7.7 mmol·L−1。考虑到色氨酸的抑制作用是针对两种不同的化合物(脯氨酸和加博沙多)测量的,并且体内转运不仅包括腔内转运,还包括体循环中的外观,其中遇到了加博沙多跨几个膜的转移,IC50值彼此惊人地接近。hPAT1底物的特点是在毫摩尔范围内具有亲和力,并且在整个肠道中表达的转运蛋白具有高容量(Chen et al., 2003)。加博沙多和色氨酸的摩尔给药比高达1:41,由于它们对hPAT1的亲和力相当,Cmax和ka的降低可能是由于加博沙多和色氨酸在吸收部位,即小肠肠细胞管腔膜上的PAT1蛋白处的竞争性相互作用。因此,最大血浆浓度随着Tmax的延长而出现,但由于容量过大和肠道中PAT1的表达,随着吸收沿着肠道的长度进行,吸收分数保持不变。因此,加博沙多的峰值血浆浓度可以通过修改吸收过程来降低,如这里所示,或者通过更经典的缓释制剂方法,如前面所建议的(Kjaer和Nielsen, 1983)。 |
| 细胞实验 |
细胞活力测定[1]
细胞类型:第 2/3 层神经元(来自雄性 C57BL/6 小鼠,出生后 13-19 天) 测试浓度: 1 µM 孵育时间: 5 s 实验结果: 诱导破伤风电流为 43.2 pA。 细胞活力测定[1] 细胞类型:第 2/3 层神经元(来自雄性 C57BL/6 小鼠,出生后 13-19 天) 测试浓度: 1 µM 孵育时间: 1 s 实验结果: 仅 mIPSC 的振幅和频率发生微小变化。 Caco-2细胞体外经上皮转运[2] 细胞培养和体外转运实验如前所述进行(Larsen等人,2008年)。简单地说,将第24 ~ 29代的细胞接种到Transwell™植入物中,并在播种后的第25 ~ 28天进行实验。在HBSS缓冲液中,测量了Gaboxadol/加博沙多在Caco-2细胞单层上从根尖到基底外侧(A-B)或基底外侧到根尖(B-A)方向的上皮转运。在所有实验中,基底外侧的HBSS的pH值为7.4,而顶部缓冲液在加入测试化合物后调整为pH 6.0或7.4。顶腔和底外侧腔体积分别为0.5和1.0 ml。在供体腔中加入新鲜的0.34、3.5或7.0 mM的加博沙多溶液开始运输实验。在18.8 mM 5-HTP存在和不存在的情况下,测定了加博沙多的转运。用18 μM [14C]-甘露醇测定了甘露醇在A-B方向的输运。分别于20、40、60、80和120 min后,从基底外侧和根尖接收室取100 μl和20 μl的样品。 经roat注射的卵母细胞摄取Gaboxadol/加博沙多[2] 在BD Gentest™运输细胞中研究了rOat1对加博沙多的摄取;预注射rOat1 cRNA的爪蟾卵母细胞。运输细胞按生产说明书处理。作为阴性对照,在注射水的卵母细胞中,即不表达rOat1的卵母细胞中,也测量了加博沙多的摄取。每个数据点5 - 10个卵母细胞放置在5ml试管中。卵母细胞用1 ml的Na+缓冲液洗涤3次,然后用100 μl的复合溶液孵育。在rOat1 cRNA注入的卵母细胞和对照卵母细胞中,分别于15、30、60、90 min和30、60、90 min取样,观察100 μM加博沙多摄取的时间依赖性。研究了10、50、100、300、500和1000 μM加博沙多在rOat1 cRNA注射的卵母细胞中60 min的摄取情况,同时研究了100、500和1000 μM加博沙多在水注射的卵母细胞中的摄取情况。测定rOat1 cRNA注射卵母细胞和水注射卵母细胞在5- htp、5-脯氨酸、1 mM PAH或1 mM probenecid存在下对100 μM加博沙多的摄取情况,孵育60 min后,卵母细胞在冰冷的Na+缓冲液中洗涤3次,每个卵母细胞置于Eppendorf管中,加入30 μl MilliQ水和25 μl内标。卵母细胞保存在- 80°C直到定量时间,通过解冻和旋转混合来裂解卵母细胞。在10,000 × g离心15分钟后,从上清中提取加博沙多,按照以下步骤进行分析:用250 μl冷(4°C)乙腈沉淀蛋白质,将干燥的样品在60 μl (30:70) MeOH/MeCN中重新溶解。 定量血浆中加博沙多/Gaboxadol和缓冲[2] 用液体萃取法从血浆和HBSS样品中提取加博沙多。将100 μl HBSS (80 μl纯净水加入20 μl样品中)或100 μl血浆样品与25 μl内标(d4-加博沙多)和25 μl纯净水混合。加入400 μl冷乙腈进行蛋白沉淀。10000 × g离心15 min后,将425 μl上清转移至玻璃管中,在45℃氮气下蒸发干燥。样品溶解于80 μl甲醇/乙腈(30:70)中,旋转混合10 min, 3000 rpm离心3 min。在盲法血浆中加入标准品,制备与血浆样品相似的标准品。采用亲水性相互作用色谱法对加博沙多进行定量,然后采用(Kall et al., 2007)改进的方案进行质谱/质谱检测。LC系统由安捷伦1100系列泵和脱气器组成。采用Phenomenex公司的Asahipak氨基柱(nh2p - 50,150 mm × 2mm),流动相为20.0 mm乙酸铵,pH值为4:乙腈(30:70),流速为0.2 ml/min。将20 μl样品进样于柱上,室温保存。总运行时间为10分钟,前5分钟的洗脱让浪费。加博沙多的洗脱时间约为8分钟。所使用的质谱联用系统包括Sciex API 4000质谱联用检测器,带有涡轮离子喷雾和涡轮V源(Applied Biosystems, CA, USA)。采用多反应监测(MRM)负电离方式检测加博沙多(前体139.1 Da,产物110.1 Da)和d4-加博沙多(前体143.0,产物112.2 Da)。在1.7 ~ 1000.0 ng/ml浓度范围内,信号呈线性关系,定量限为1.7 ng/ml。软件来自Analyst™。 细胞培养和体外实验方案如前所述(Larsen等人,2008年)。将第20 ~ 29代Caco-2细胞接种到Transwell™插入物(1.12 cm2, 0.4µm孔径)上,于接种后第25 ~ 28天进行实验。在汉克斯平衡盐溶液(HBSS)缓冲液中,测定了Gaboxadol/加博沙多在Caco-2细胞单分子层从根尖向基底侧方向(A-B)和基底侧向根尖方向(B-A)的顶端摄取和上皮转运。在所有实验中,基底外侧的缓冲液pH为7.4。除非另有说明,在加入Gaboxadol/加博沙多盐酸或35 mmol·L−1色氨酸后,将应用于顶室的缓冲液调至pH 6.0。研究了0.34、3.5或7.0 mmol·L−1Gaboxadol的转运情况。这些浓度的选择是基于人单次睡前口服15mg加博沙多,提供约0.34 mmol·L−1的通径浓度,以及所获得的加博沙多对hPAT1的亲和力。在根尖室中加入含有12.5 nmol·L−1(0.5µCi) L-(3H)脯氨酸和0-30 mmol·L−1加博沙多或0-35 mmol·L−1色氨酸的新鲜根尖HBSS培养基,进行根尖摄取实验。5 min后终止根尖摄取实验,对样品进行闪烁计数分析。[6] |
| 动物实验 |
Animal/Disease Models: SD (SD (Sprague-Dawley)) rat (255-276 g) [2].
Doses: 2.5 mg/kg (intravenous (iv) (iv)injection); 0.5 and 5.0 mg/kg (oral). Route of Administration: po (oral gavage); intravenous (iv) (iv)injection; single. Experimental Results: 1.19 pharmacokinetic/PK/PK parameters of THIP 4 in SD (SD (Sprague-Dawley)) rats [2]. IV (2.5 mg/kg) PO (0.5 mg/kg) PO (5.0 mg/kg) AUC (ng/mL·h) 1193 263 1988 Tmax (h) - 0.22 0.33 Cmax (ng/mL) 4350 291 2061 CL/ Fa (mL/h/kg) - 1939 2558 CL (mL/h/kg) 2043 - - Fa (%) 100 110 83 In vivo absorption of Gaboxadol in Sprague–Dawley rats [2] Male Sprague–Dawley rats were housed and acclimated for 7 days before the experiments. The animals weighed 255–276 g at the day of the experiment. The rats were maintained on standard food and water until 16–20 h prior to dosing where food was retrieved. Water was available to the animals until beginning of experiment and again 2 h after dosing. Each animal was randomly assigned to receive one of the intravenous or oral formulations, in total 6 parallel groups of 6 animals (n = 6) unless otherwise stated. The oral solutions contained 0.5 or 5.0 mg/kg of gaboxadol and the intravenous solutions contained 2.5 mg/kg gaboxadol. The animals were orally dosed with 10.0 ml/kg (i.e. concentrations of 0.35 and 3.5 mM gaboxadol) or intravenously with 5 ml/kg (3.5 mM). The gaboxadol doses were given 30 min after an oral pre-dose of isotonic saline or 100.0 mg/kg 5-HTP (for IV gaboxadol) or 200.0 mg/kg 5-HTP (for oral gaboxadol) by oral gavage (10.0 ml/kg). All solutions were adjusted to a pH of 5.2 before the osmolarity was checked on a Vapro vapour pressure osmometer and adjusted with mannitol to iso-osmolarity. Blood samples (0.2 ml) were taken from the tail vein by individual vein puncture and collected into Eppendorf tubes containing 20 IE heparin. Blood samples were collected at 5, 15, 30, 45, 60 min and 2, 3, 4, 6, 8 h after gaboxadol administration. The plasma was harvested immediately by centrifugation for 10 min at 3600 × g and stored at −80 °C until further analysis. After the experiment the animals were euthanized. Investigation of gastric emptying in rats [5] A protocol similar to the one described above using paracetamol as a marker was used to investigate paracetamol absorption and evaluate the influence of 5-HTP on the gastric emptying rate in rats. 26 rats were selected and allocated randomly to receive one of 5 different formulations of paracetamol. The rats received 120.0 mg/kg paracetamol and 2.5 mg/kg Gaboxadol (10 ml/kg) as an intravenous injection or oral solution. The oral solutions were given 30 min after an oral pre-dose of isotonic solutions of saline, mannitol or 200 mg/kg 5-HTP. The intravenous injection of gaboxadol was given 30 min after an oral pre-dose of isotonic saline (n = 6) or 200.0 mg/kg 5-HTP (n = 2). Drug administration, blood sampling and handling of animals was done as described above. Animal/Disease Models: Fmr1 KO2 mice (the Fmr1 promoter and first exon are deleted, resulting in mice with missing mRNA and protein) [5] Doses: 0.5, 1, 1.5, 2, 3, 4 or 5 mg/kg given Medication: intraperitoneal (ip) injection Experimental Results: Normalized hyperactivity was observed in Fmr1 KO2 mice. Mice in the same cage were injected with the same dose of Gaboxadol or vehicle, and mutants and controls were housed separately. All mice were group-housed in plastic cages (35 × 30 × 12 cm), five per cage, and habituated to the animal facility for at least a week before testing. The room temperature (21 ± 2°C), relative humidity (55 ± 5%), a 12 h light-dark cycle (lights on 7 am–7 pm) and air exchange (16 times per hour) were automatically controlled. All mice had ad libitum access to food and water. All testing was conducted in the light-phase by an investigator blind to genotype and drug treatment. [2] Gaboxadol Treatment and Experimental Timeline: Fmr1 KO2 mice were injected with vehicle (0.9% sterile saline) or Gaboxadol (0.5, 1, 1.5, 2, 3, 4, or 5 mg/kg, i.p.) 30 min prior to behavioral testing on each testing day, with a three-day interval between each test to avoid any cumulative effect of the drug administration. Wild-type mice injected with vehicle at the same time point were also included in all experiments. Behavioral screening of the mice (n = 10 per group) was conducted in the following order with 2–3 days between each test: Open Field Test (OFT; day 1), successive alleys (day 4), light/dark box (day 7), social tests and aggression (day 10), and self-grooming and stereotypy (day 12). [5] Absorption of Gaboxadol in dogs [6] All animal care and experimental studies were approved by the Animal Welfare Committee, appointed by the Danish Ministry of Justice, and were carried out in compliance with EC Directive 86/609/EEC, the Danish law regulating experiments on animals and NIH Guidelines for the Care and Use of Laboratory Animals. Six full-grown male beagle dogs (body weight 15.9–21.7 kg) were selected and allocated into a Roman quadrant design and assigned to receive all the six formulations of Gaboxadol hydrochloride randomly during 6 weeks. The dogs were fasted for 20–24 h before the initiation of the experiment and fed again 10 h after the administration. The gaboxadol dose was given either as an intravenous injection (1.0 mL·kg−1) or as an oral solution given by gavage (5.0 mL·kg−1) directly into the stomach using a soft tube. All dogs received 2.5 mg·kg−1 gaboxadol. In addition to gaboxadol, the oral formulations contained 0, 2.5, 10, 50 or 150 mg·kg−1 of tryptophan to ensure simultaneous co-administration of the two compounds. All solutions were adjusted to a pH of 5.2, and osmolarity was checked with a Vapro vapor pressure osmometer (model 552O, Wescor Inc., Logan, UT, USA), the intravenous solutions were adjusted to iso-osmolarity with glucose. Blood samples (2 mL) were taken from the cephalic vein by individual venepuncture and collected into Eppendorf tubes containing 200 IE heparin as an anticoagulant. Samples were collected before administration of gaboxadol and after 5, 15, 30, 60, 90 min, and 2, 3, 4, 6, 8 and 10 h after gaboxadol administration. The plasma was harvested immediately by centrifugation for 15 min at 2200 g and 4–8°C and stored at −80°C until further analysis. The animals had a 6-day washout period between treatments. Investigation of gastric emptying in dog [6] A protocol similar to the one described earlier using paracetamol as a marker was used to evaluate the influence of tryptophan on the gastric emptying rate in dogs. Six dogs (body weight 16.1–21.5 kg) were selected and randomly allocated to receive three formulations of paracetamol in a crossover study. The dogs received 50 mg·kg−1 paracetamol as an intravenous injection (1 mL·kg−1) or as an oral solution (5 mL·kg−1) containing 2.5 mg·kg−1 Gaboxadol and 0 or 150 mg·kg−1 tryptophan. Fasting of the dogs, drug administration, blood sampling and washout were done as described earlier. Analytical methods [6] Quantification of Gaboxadol in plasma and buffer: Gaboxadol was extracted from plasma and buffer samples by liquid extraction. 100 µL HBSS or plasma samples were mixed with 25 µL internal standard (d4-gaboxadol) and 25 µL purified water. Protein precipitation was carried out by addition of 400 µL cold acetonitrile. After centrifugation at 10 000 g for 15 min, 425 µL of supernatant was transferred to glass tubes and evaporated to dryness under nitrogen at 45°C. The samples were redissolved in 80 µL of methanol/acetonitrile (30:70), whirl-mixed for 10 min and centrifuged for 3 min at 3300× g. Gaboxadol was subsequently quantified by hydrophilic interaction chromatography followed by tandem mass spectrometry (MS/MS) detection using a protocol modified from Kall et al. (2007). The liquid chromatography (LC) system comprised by an Agilent 1100 series pump and degasser. An Asahipak amino column, (NH2P-50, 150 × 2 mm) from Phenomenex (Torrance, CA, USA) was used with a mobile phase of 20.0 mmol·L−1 ammonium acetate (pH 4): acetonitrile (30:70) and a flow rate of 0.2 mL·min−1. Twenty-microlitre samples were injected onto the column, which was kept at room temperature. The total run time was 10 min with the first 5 min of elution let to waste. The elution time of gaboxadol on the column was approximately 8 min. The MS/MS system used consisted of a Sciex API 4000 MS/MS detector with a Turbo Ion Spray and Turbo V source (Applied Biosystems, Foster City, CA, USA). The signals were linear between 0.5 and 2500 ng·mL−1, and the limit of quantification by this procedure was 0.5 ng·mL−1. The software was from Analyst™ (Applied Biosystems, version 4.0). |
| 药代性质 (ADME/PK) |
Pharmacokinetic analysis of oral Gaboxadol absorption in Sprague–Dawley rats [2]
The gaboxadol plasma concentration profiles after intravenous and oral administration to Sprague–Dawley rats are shown in Fig. 1. The time to maximum plasma concentration, Tmax, was 0.2 and 0.3 h for the dose groups of 0.5 and 5.0 mg/kg gaboxadol, respectively (Table 2). The oral absorption of 0.5 mg/kg gaboxadol appeared to be complete as the mean bioavailability, Fa, was 110%. The Fa of 5 mg/kg was 83 ± 5%, which is significant lower than the Fa determined for 0.5 mg/kg (p < 0.01). The clearance, CL/Fa, of gaboxadol increased from 1.93 to 2.56 l/h/kg for the doses of 0.5 and 5 mg/kg gaboxadol (p < 0.05), respectively. The peak plasma concentration, Cmax, increased 7 times when the dose increased 10-fold from 0.5 to 5 mg/kg and did not indicate dose proportionality at the investigated dosage range. Furthermore, the AUC only increased 7.5 times after a 10-fold increase in dose. The nonlinear pharmacokinetics suggested that a transport process in the absorption or the elimination of gaboxadol was carrier mediated. The carrier could be a saturable absorptive carrier in the intestine or a carrier involved in reabsorption of gaboxadol in the kidney. Administration of oral Gaboxadol and 5-HTP to Sprague–Dawley rats [2] 5-HTP administration significantly changed the gaboxadol plasma concentration profiles after both intravenous and oral dosing in rats (Fig. 1A–C) The absorption and elimination phases were altered, furthermore the AUC was significantly increased as a consequence of pre-dosing with 5-HTP in rats. During the initial absorption phase of gaboxadol (≤Tmax) the gaboxadol plasma concentrations with 5-HTP dosing were significantly lower as compared to the plasma concentrations in the animals not receiving 5-HTP (p < 0.05, Fig. 1B and C). 5-HTP did not significantly change the maximal plasma concentration, Cmax, of gaboxadol. Furthermore, 5-HTP caused a significant prolongation of the Tmax (p < 0.01 and p < 0.001) for 0.5 and 5.0 mg/kg, respectively, indicating that 5-HTP alters the intestinal absorption of gaboxadol in rats. The gaboxadol elimination phases shown in the semilogarithmic plots in Fig. 1A and B were approximately straight lines for rats dosed with 5-HTP and biphasic curves for gaboxadol administered alone. The presence of 5-HTP increased the mean AUC of 0.5 and 5.0 mg/kg gaboxadol by a factor of 5.5 (p < 0.01) and 3.6, respectively. The mean clearance, CL/Fa, of 0.5 and 5.0 mg/kg gaboxadol was decreased by 77% (p < 0.01) and 23% (not significant), respectively. The clearance, CL, of 2.5 mg/kg (IV) gaboxadol was decreased by and 66% (n = 2) in the presence of 5-HTP (Table 2). The oral absorption of paracetamol, used in the present study as a marker for gastric emptying, was not significantly changed after pre-dosing with 5-HTP or mannitol based on an evaluation of Cmax, Tmax, AUC, Fa and, CL/Fa (Fig. 2 and Table 3). Therefore, it is concluded that 5-HTP did not significantly prolong the gastric emptying. However, the elimination rate constant for paracetamol, ke (0.95 h−1), was significant lower in the presence of 5-HTP (0.53 h−1, p < 0.05). Gaboxadol is a substrate of rOat1, but 5-HTP is not [2] Since 5-HTP had a significant effect on gaboxadol clearance in rats, the transport of gaboxadol by the renal organic anion transporter, rOat1 was investigated in the absence or presence of 5-HTP (Fig. 3, Fig. 4). The uptake of gaboxadol in both rOat1 cRNA and water injected oocytes was linear over the 90 min investigated (Fig. 3A). The gaboxadol uptake was significantly higher in rOat1 cRNA than in water injected oocytes (p ≤ 0.001). The concentration dependent uptake rate of gaboxadol in rOat1 cRNA injected oocytes was saturable (Fig. 3B). The data points could be described by Michaelis–Menten kinetics with a Km of 151.2 ± 58.19 μM and a Vmax of 0.78 ± 0.09 pmol oocyte−1 min−1. Gaboxadol is thus a substrate for rOat1 and we therefore investigated the rOat1-mediated uptake of gaboxadol in the presence of 5 mM 5-HTP, 5 mM l-proline, 1 mM PAH and 1 mM probenecid (Fig. 4). The uptake of gaboxadol was not changed by the presence of 5-HTP or l-proline, whereas the uptake of gaboxadol in the presence of PAH and probenecid (substrate and inhibitor of Oat1, respectively) was significantly reduced (p < 0.001). 5-HTP decreases Gaboxadol absorption in vitro and in vivo [2] Transport of gaboxadol across Caco-2 cell monolayers was polarized in the A–B direction. The A–B gaboxadol transport was dependent on donor concentration and pH in the donor medium. This is consistent with gaboxadol transport across the luminal membrane of enterocytes mediated by hPAT1, a transport step which appears to be important for the resulting transepithelial absorption. Similar observations have been made in Caco-2 cell monolayers regarding the pH dependent transport of other PAT1 substrates such as l-proline (Thwaites et al., 1993) and GABA (Thwaites et al., 2000). Previously, hPAT1 was cloned from Caco-2 cells by Chen et al. and immunofluorescence showed that PAT1 is expressed at the apical membrane of the Caco-2 cells and of the small intestinal enterocytes in rats (Chen et al., 2003, Anderson et al., 2004). In Caco-2 cell monolayers 80–90% of gaboxadol transport was most likely mediated by hPAT1 and 5-HTP inhibited this transport. The Ki-values of gaboxadol and 5-HTP for inhibition of l-proline uptake in Caco-2 cells via hPAT1 were 6.6 mM (Larsen et al., 2009) and 2.3 mM, respectively (Larsen et al., 2008). The question is thus if the interaction between gaboxadol and 5-HTP is observed at relevant concentrations. Assuming an oral gaboxadol dose of 15 mg and a volume of 250 ml the resulting luminal concentration is 0.34 mM. For 5-HTP a normal dose is 50–100 mg resulting in a luminal concentration of 9–18 mM. In our experiment the lowest concentration of gaboxadol is 0.34 mM and that of 5-HTP is 18.8 mM. The concentrations are thus possible to obtain in vivo and the interaction between the two compounds likely to be relevant. In vivo the peak plasma concentration of gaboxadol was reached within 0.22–0.33 h after oral administration to Sprague–Dawley rats. During the initial absorption phase of gaboxadol, significantly lower plasma gaboxadol concentrations were observed in the rats pre-dosed with 5-HTP compared to the rats dosed only with gaboxadol. Furthermore, the presence of 5-HTP significantly increased Tmax of gaboxadol. A parallel study of paracetamol absorption, an often used marker for gastric emptying, showed that 5-HTP did not significantly delay the gastric emptying. This indicated that 5-HTP decreased the absorption rate of gaboxadol in the rat small intestine. Taken together with the in vitro findings of hPAT1-mediated transepithelial transport of gaboxadol, it is suggested that 5-HTP decreased the absorption rate of gaboxadol by its interaction with PAT1 in the rat small intestine. 4.2. Renal excretion of gaboxadol is likely to be dependent on transporters and the presence of 5-HTP In addition to decreasing the initial absorption of Gaboxadol, 5-HTP also increased AUC, mainly due to the changed the plasma profile of gaboxadol after Tmax. Previous studies have shown that gaboxadol has a low degree of plasma protein binding (Lund et al., 2006) furthermore, it is not a substrate of cytochrome P-450's (Schultz et al., 1981, Lund et al., 2006). Consequently, a decrease in gaboxadol clearance was likely to account for the increased gaboxadol AUC. The significantly prolonged Tmax is consistent with a decreased gaboxadol clearance however; Cmax was not significantly changed by 5-HTP pre-dosing. Perhaps, the simultaneous inhibition of gaboxadol absorption rate via rPat1 and a reduced gaboxadol clearance can explain the steady Cmax level observed. Gaboxadol is excreted predominantly via the kidneys, and variable amounts of metabolites can be found in urine i.g. in human urine, 34% of gaboxadol was found as an O-glucuronide (Lund et al., 2006). In rat urine, however, only 2–7% O-glucuronide has been found, and accordingly changes in metabolism do not seem to affect the results obtained the present study (Schultz et al., 1981). Subsequent to identifying rPat1 as the most likely responsible intestinal transporter of Gaboxadol, we looked for transporters that could participate in renal handling of gaboxadol. The proton-coupled amino acid transporters rPat1 and rPat2 could be involved in reabsorption of gaboxadol from the urine, however the effect of 5-HTP would then likely be decreasing the reabsorption leading to an increased renal excretion and clearance. After 5-HTP pre-dosing the opposite was observed, i.e. a decrease in CL and CL/Fa, which points to a possible inhibition of an efflux transporter in the apical membrane or an influx transporter in the basolateral membrane of rat renal epithelial cells. A previous study on the pharmacokinetics and elimination of acyclovir in humans found similar effects of an increased AUC and a decreased drug excretion rate when the patients were also administered with probenecid (Laskin et al., 1982). As probenecid is an inhibitor of efflux transporters such as OAT1, and acyclovir is a substrate of rOat1, an interaction at hOAT1 most likely resulted in a decreased tubular secretion of the drug (Wada et al., 2000). Furthermore, it was recently suggested that the transport of gaboxadol into the human kidney happens via hOAT1 (Chu et al., 2009). Therefore, the rat basolateral renal organic anion transporter 1, rOat1, was investigated and it was demonstrated that gaboxadol is a substrate, whereas 5-HTP is not. Organic anion transporters are characterized by a broad substrate specificity and have been shown to transport especially small amphiphilic molecules across boundary epithelia (Rizwan and Burckhardt, 2007). The gaboxadol uptake rate in rOat1 cRNA injected oocytes was characterized by a mean Km value of 151 μM. This value is quite similar to the Km value of gaboxadol uptake (115 ± 27 μM) obtained in hOAT1 transfected CHO-K1 cells (Chu et al., 2009). In comparison, most antibiotics have lower affinities for hOAT1, whereas NSAIDs have higher affinities classifying gaboxadol as a medium affinity substrate for rOat1 (Apiwattanakul et al., 1999, Rizwan and Burckhardt, 2007). The murine and human orthologues of rOat1, mOat1 and hOAT1, respectively, have been observed to facilitate the transport of neuroactive tryptophan metabolites (Alebouyeh et al., 2003, Bahn et al., 2005). However, the uptake of gaboxadol in oocytes via rOat1 was not decreased by the presence of 5-HTP indicating that 5-HTP does not directly inhibit gaboxadol excretion via rOat1. Nevertheless, in rats, 5-HTP can be metabolised by the aromatic l-amino acid decarboxylase (LAAD) to 5-hydroxytryptamine (serotonin or 5-HT) and further by monoamine oxidase (MAO) to 5-hydroxyindoleacetic acid (5-HIAA) (Stier et al., 1984, Wang et al., 2001). 1 mM 5-HIAA has been reported to inhibit the uptake of 0.25 μM [3H]-PAH in mOat1 transfected COS-7 cells with approximately 90% (Bahn et al., 2005). Thus, depending on the formation rate of 5-HIAA in rats and affinity values of the compounds, interaction of 5-HIAA with gaboxadol at rOat1 might be possible. The maximal Gaboxadol plasma concentration observed following doses of 5.0 mg/kg in the present in vivo study was approximately 2000 ng/ml, which corresponds to 14.3 μM. Thus, the clearance of gaboxadol was probably not limited by the capacity of rOat1. The effects on the clearance of gaboxadol observed with 5-HTP cannot be accounted for in terms of the transporters identified here. A possible explanation might be found in the effects of 5-HT on renal blood flow and glomerular filtration rate. In isolated perfused rat kidneys as well as in rat kidneys in vivo, 5-HT has been shown to mediate selective constriction of the afferent glomerular arterioles and significantly alter the renal function (Stier et al., 1984, Ding et al., 1989, Wang et al., 2001). In anesthetized rats, the glomerular filtration rate, effective plasma flow and urine flow were decreased by 26–41% during a 20 min infusion of 15 and 75 μg/min 5-HTP (Stier and Itskovitz, 1985). This would also account for the decreased paracetamol elimination rate constant observed in the present study, and generally for compounds with a certain extent of renal excretion, although interactions at Oat1 cannot be ruled out (Khamdang et al., 2002). [2] Conclusion The present study demonstrated that Gaboxadol transport across Caco-2 cell monolayers is most likely mediated via PAT1 and that 5-HTP inhibits this transport. The oral absorption of gaboxadol in rats might also be rPat1-mediated as the initial gaboxadol absorption was decreased by the presence of 5-HTP. The anion transporter rOat1 is suggested to be involved in the renal handling of gaboxadol in rats. The present study illustrates a possible drug–dietary supplement interaction as 5-HTP significantly changed the overall pharmacokinetic profile of gaboxadol. Although it is difficult to speculate about the consequences in humans taking gaboxadol for the treatment of insomnia or another indication along with 5-HTP supplements it could cause severe side effects. The identification of transporters involved in determining the pharmacokinetic profile of gaboxadol provides the basis for a further understanding of potential drug–drug interactions. Pharmacokinetic analysis of oral Gaboxadol absorption in dog [6] Gaboxadol plasma concentration profiles following oral or intravenous administration of 2.5 mg·kg−1 gaboxadol in beagle dogs were monitored over 10 h (Figure 3). The bioavailability, Fa, of gaboxadol following oral administration in dog was high (over 80%) (Table 1). Oral co-administration of 2.5–150 mg·kg−1 tryptophan did not change the AUC of gaboxadol significantly, and the mean relative bioavailability of the formulations varied between 75 (10 mg·kg−1 tryptophan) and 86.1% (2.5 mg·kg−1 tryptophan). Also, the elimination rate constant (ke) and the clearance (CL) of gaboxadol did not change with co-administration of tryptophan. However, co-administration of 150 mg·kg−1 tryptophan decreased the maximal gaboxadol plasma concentration, Cmax, from 2502 to 1419 ng·mL−1, that is, 57%. Furthermore, the time required to reach the maximal plasma concentration, Tmax, was increased from 0.46 h to 1.5 h (P < 0.01). The Cmax values of the five dose groups were subsequently fitted to a dose-response curve (Figure 4), which indicated a direct interaction between gaboxadol absorption and tryptophan concentration. The in vivo IC50 value of tryptophan on gaboxadol Cmax was estimated to be 12.6 mg·kg−1, which is equivalent to a concentration of 12.3 mmol·L−1 tryptophan (not corrected for dilution in gastric and intestinal fluids). Absorption rate constants of Gaboxadol and paracetamol [6] Co-administration of increasing tryptophan doses gradually changed the mean cumulative fraction of absorbed gaboxadol as seen in the deconvolution profiles in Figure 5A. The absorption of gaboxadol in the presence of 150 mg·kg−1 tryptophan was significantly decreased at time points 0.5–1.25 h compared with the absorption of gaboxadol alone. An oral dose of 91.5 ± 3.3% of paracetamol was absorbed after 60 min (Figure 5B) indicating that gastric emptying happens mainly within the first hour after administration. Co-administration of 150.0 mg·kg−1 of tryptophan did not significantly change the gastric emptying rate, as the fraction of absorbed paracetamol in the absence or presence of tryptophan was not significantly different at the time points tested. The pharmacokinetic parameters Tmax, AUC and CL of plasma paracetamol concentrations were not significantly different from parameters obtained after co-administration of paracetamol and tryptophan (results not shown). Based on the profiles shown in Figure 5A, the absorption rate constant, ka, of gaboxadol were calculated and these are depicted as a function of the logarithmic tryptophan dose in Figure 6A. The ka of gaboxadol was decreased by co-administration of tryptophan with an in vivo IC50 value on gaboxadol absorption of 10.3 mg·kg−1, which corresponds to an oral solution with a concentration of 10.1 mmol·L−1 tryptophan. Figure 6B shows that 150 mg·kg−1 of the PAT1 inhibitor tryptophan significantly decreased the absorption rate constant of gaboxadol (P < 0.01), whereas it had no significant effect on the absorption rate constant of paracetamol. |
| 毒性/毒理 (Toxicokinetics/TK) |
mouse LD50 intraperitoneal 98 mg/kg SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE; BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); SKIN AND APPENDAGES (SKIN): HAIR: OTHER Neuropharmacology., 21(803), 1982 [PMID:7121752]
|
| 参考文献 |
|
| 其他信息 |
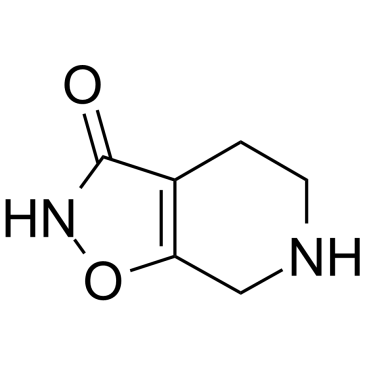
4,5,6,7-Tetrahydroisoxazolo(5,4-c)pyridin-3-ol is an oxazole.
Gaboxadol also known as 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP) is an experimental sleep aid drug developed by Lundbeck and Merck, who reported increased deep sleep without the reinforcing effects of benzodiazepines. Development of Gaboxadol was stopped in March 2007 after concerns regarding safety and efficacy. It acts on the GABA system, but in a seemingly different way from benzodiazepines and other sedatives. Drug Indication Investigated for use/treatment in sleep disorders and insomnia. We have studied the actions of the δ-subunit preferring hypnotic drug THIP on neuronal GABAA receptors in the mouse neocortex. We found that Gaboxadol/THIP, at clinically relevant concentrations, induced a significant GABAA-mediated tonic current in neocortical neurons, without affecting intrasynaptic GABAA receptors. The magnitude of the tonic current correlated qualitatively with the δ-subunit expression in neocortical layers. Surprisingly, THIP caused a pronounced decrease in the frequency of activity driven sIPSCs in layer 2/3 neurons, while the frequency of sEPSCs increased. These results indicate that THIP primarily acts at extrasynaptic GABAA receptors in the neocortex, and may particularly depress interneuron activity to diminish inhibitory synaptic input. This may explain why THIP alters the cortical oscillatory activity (Vyazovskiy et al., 2005), since the spike timing, in part, depends on the networks of extensively connected interneurons (Tamas et al., 2000; Buhl et al., 1998).[1] GABA(C) receptors are being investigated for their role in many aspects of nervous system function including memory, myopia, pain and sleep. There is evidence for functional GABA(C) receptors in many tissues such as retina, hippocampus, spinal cord, superior colliculus, pituitary and the gut. This review describes a variety of neurochemicals that have been shown to be useful in distinguishing GABA(C) receptors from other receptors for the major inhibitory neurotransmitter GABA. Some selective agonists (including (+)-CAMP and 5-methyl-IAA), competitive antagonists (such as TPMPA, (±)-cis-3-ACPBPA and aza-THIP), positive (allopregnanolone) and negative modulators (epipregnanolone, loreclezole) are described. Neurochemicals that may assist in distinguishing between homomeric ρ1 and ρ2 GABA(C) receptors (2-methyl-TACA and cyclothiazide) are also covered. Given their less widespread distribution, lower abundance and relative structural simplicity compared to GABA(A) and GABA(B) receptors, GABA(C) receptors are attractive drug targets. [4] Gaboxadol normalized all of the tested behavioral deficits of Fmr1 KO2 mice at a dose of 0.5 mg/kg. While higher doses also normalized irritability and aggressive behaviors, this was not observed for other behavioral domains evaluated. One explanation for the somewhat narrow efficacy window observed here may come from previous work showing compromised information processing by either insufficient or excess tonic inhibition, the physiological process that Gaboxadol potentiates. Under this model, the behavioral benefit of drug at high doses would be offset by pharmacologically introduced FXS-independent deficits (Duguid et al., 2012). Our results provide robust evidence of the potential benefit of Gaboxadol in reversing ASD related behaviors, aggression and sociability. Taken together, these results support the hypothesis that potentiation of extrasynaptic GABAA receptors by gaboxadol may be of benefit in individuals with FXS. In conclusion, these data support the future evaluation of gaboxadol in individuals with FXS, particularly with regard to symptoms of hyperactivity, anxiety, ASD related stereotypy, sociability, irritability, aggression, and cognition.[5] Background and purpose: Gaboxadol has been in development for treatment of chronic pain and insomnia. The clinical use of Gaboxadol has revealed that adverse effects seem related to peak serum concentrations. The aim of this study was to investigate the mechanism of intestinal absorption of gaboxadol in vitro and in vivo. Experimental approach: In vitro transport investigations were performed in Caco-2 cell monolayers. In vivo pharmacokinetic investigations were conducted in beagle dogs. Gaboxadol doses of 2.5 mg.kg(-1) were given either as an intravenous injection (1.0 mL.kg(-1)) or as an oral solution (5.0 mL.kg(-1)). Key results: Gaboxadol may be a substrate of the human proton-coupled amino acid transporter, hPAT1 and it inhibited the hPAT1-mediated L-[(3)H]proline uptake in Caco-2 cell monolayers with an inhibition constant K(i) of 6.6 mmol.L(-1). The transepithelial transport of gaboxadol was polarized in the apical to basolateral direction, and was dependent on gaboxadol concentration and pH of the apical buffer solution. In beagle dogs, the absorption of gaboxadol was almost complete (absolute bioavailability, F(a), of 85.3%) and T(max) was 0.46 h. Oral co-administration with 2.5-150 mg.kg(-1) of the PAT1 inhibitor, L-tryptophan, significantly decreased the absorption rate constant, k(a), and C(max), and increased T(max) of gaboxadol, whereas the area under the curve and clearance of gaboxadol were constant. Conclusions and implications: The absorption of Gaboxadol across the luminal membrane of the small intestinal enterocytes is probably mediated by PAT1. This knowledge is useful for reducing gaboxadol absorption rates in order to decrease peak plasma concentrations.[6] In conclusion, the present study shows for the first time that the high permeability of Gaboxadol across Caco-2 cell monolayers is most likely due to PAT1-mediated transport across the luminal membrane resulting in a high transepithelial transport. The in vitro gaboxadol transport kinetics and the pharmacokinetics observed in dogs support the conclusion that PAT1 mediates transport of gaboxadol across the mucosal membrane both in vitro as well as in vivo. In addition, the present study indicates that it is possible to exploit transporter activity in order to modify or control the intestinal absorption of drug substances. The formulation design provides a simple basis for decreasing peak plasma concentration of gaboxadol, while maintaining a high bioavailability. This may aid in reducing side effects related to high plasma peak concentrations.[6] |
| 分子式 |
C₆H₈N₂O₂
|
|---|---|
| 分子量 |
140.14
|
| 精确质量 |
140.058
|
| CAS号 |
64603-91-4
|
| 相关CAS号 |
Gaboxadol hydrochloride;85118-33-8
|
| PubChem CID |
3448
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
340.5±42.0 °C at 760 mmHg
|
| 闪点 |
159.7±27.9 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.551
|
| LogP |
-0.61
|
| tPSA |
58.29
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
210
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1CNCC2=C1C(=O)NO2
|
| InChi Key |
ZXRVKCBLGJOCEE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H8N2O2/c9-6-4-1-2-7-3-5(4)10-8-6/h7H,1-3H2,(H,8,9)
|
| 化学名 |
4,5,6,7-Tetrahydroisoxazolo(5,4-c)pyridin-3(2H)-one4,5,6,7-Tetrahydro-[1,2]oxazolo[5,4-c]pyridin-6-ium-3-one
|
| 别名 |
Gaboxadol; OV-101; MK0928; Lu02030; OV101; Lu-02-030; gaboxadol; 64603-91-4; Gaboxadolum; Gaboxadolum [Latin]; Gaboxadol [USAN:INN]; 4,5,6,7-Tetrahydroisoxazolo(5,4-c)pyridin-3-ol; Lu 02-030; MK-0928; Lu-02030
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~356.79 mM)
H2O : ~23.33 mg/mL (~166.48 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (17.84 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (17.84 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (14.84 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50 mg/mL (356.79 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.1357 mL | 35.6786 mL | 71.3572 mL | |
| 5 mM | 1.4271 mL | 7.1357 mL | 14.2714 mL | |
| 10 mM | 0.7136 mL | 3.5679 mL | 7.1357 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00209963 | Completed | Drug: Gaboxadol | Primary Insomnia | H. Lundbeck A/S | 2003-06 | Phase 3 |
| NCT06334419 | Recruiting | Drug: Gaboxadol Drug: Placebo |
Fragile X Syndrome | Craig Erickson | 2024-01-29 | Phase 2 |
| NCT00209846 | Completed | Drug: Gaboxadol | Primary Insomnia | H. Lundbeck A/S | 2004-06 | Phase 3 |
| NCT00209924 | Completed | Drug: Gaboxadol | Primary Insomnia | H. Lundbeck A/S | 2004-04 | Phase 3 |
| NCT02996305 | Completed | Drug: OV101 Regimen 1 Drug: OV101 regimen 2 Other: Placebo |
Angelman Syndrome | Ovid Therapeutics Inc. | 2016-01 | Phase 2 |